Ecomorphological Disparity in an Adaptive Radiation: Opercular Bone Shape and Stable Isotopes in Antarctic Icefishes Laura A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Examples of Amino Acid Changes in Notothenioids Methods To
Supplementary Information S1 Candidate genes: examples of amino acid changes in notothenioids Methods To evaluate notothenioid amino acid changes in a cross‐section of genes, six candidates from a range of functional categories of interest were chosen for a more detailed examination. The selected genes comprised small, well‐characterised genes present in multiple fish species (mainly teleosts, but in some cases these analyses were extended to the Actinopterygii when sequences from the spotted gar (Lepisosteus oculatus) were available) and available in at least two notothenioids. The amino acid composition of a series of six candidate genes were further analysed in depth at the amino acid sequence level. These genes were superoxide dismutase 1 (SOD1), neuroglobin, dihydrofolate reductase (both paralogues), p53 and calmodulin. Clustal X alignments were constructed from orthologues identified in the four Notothenioid transcriptomes along with other fish orthologues extracted from either SwissProt (Bateman et al., 2017) or Ensembl release 91 (Aken et al., 2017). Alignment data were visualised and annotated in BoxShade version 3.21 (https://embnet.vital‐ it.ch/software/BOX_form.html). Notothenioid‐specific changes were noted and the type of substitution in terms of amino acid properties was noted. Results A range of amino acid changes were revealed, from virtually complete conservation between the notothenioids and other fish (calmodulin), to one amino acid substitution present in neuroglobin and SOD1, to more extensive changes in dihydrofolate reductase, dihydrofolate reductase‐like and p53. In the case of the latter three genes, notothenioid‐specific changes were at positions that were often highly variable in temperate fish species with up to six different amino acid substitutions and did not result in a definable pattern of directional substitution. -

A GUIDE to IDENTIFICATION of FISHES CAUGHT ALONG with the ANTARCTIC KRILL Author(S) 1) Iwami, T
Document No. [ to be completed by the Secretariat ] WG-EMM-07/32 Date submitted [ to be completed by the Secretariat ] 1 July 2007 Language [ to be completed by the Secretariat ] Original: English Agenda Agenda Item No(s): 4.3 Title A GUIDE TO IDENTIFICATION OF FISHES CAUGHT ALONG WITH THE ANTARCTIC KRILL Author(s) 1) Iwami, T. and 2) M. Naganobu Affiliation(s) 1) Laboratory of Biology, Tokyo Kasei Gakuin University 2) National Research Institute of Far Seas Fisheries Published or accepted for publication elsewhere? Yes No x If published, give details ABSTRACT A field key to early life stages of Antarctic fish caught along with the Antarctic krill is produced. The key includes 8 families and 28 species mainly from the Atlantic sector of the Southern Ocean and uses distinguished characters which permit rapid field identification. In some cases, however, it is impossible to discriminate among species of the same family by remarkable characters. A species key is not shown for such resemble species and a brief summary of the main morphological features of species and genera is provided. SUMMARY OF FINDINGS AS RELATED TO NOMINATED AGENDA ITEMS Agenda Item Finding 4.3 We are producing a practical field key to juvenile fish caught along with the Antarctic Scientific krill. To our knowledge more than 40 species of fish have been found as by-catch. Observation However, the number of dominant fish species found in the krill catch never exceeds 20 species. An useful and practical identification key to these dominant species maybe facilitate the quantitative assessment of fish in the krill catch. -

Bony Fish Guide
This guide will help you to complete the Bony Fish Observation Worksheet. Bony Fish Guide Fish (n.) An ectothermic (cold-blooded) vertebrate (with a backbone) aquatic (lives in water) animal that moves with the help of fins (limbs with no fingers or toes) and breathes with gills. This definition might seem very broad, and that is because fish are one of the most diverse groups of animals on the planet—there are a lot of fish in the sea (not to mention rivers, lakes and ponds). In fact, scientists count at least 32,000 species of fish—more than any other type of vertebrate. Fish are split into three broad classes: Jawless Fish Cartilaginous Fish Bony Fish (hagfish, lampreys, etc.) (sharks, rays, skates, etc.) (all other fish) This guide will focus on the Bony Fish. There are at least 28,000 species of bony fish, and they are found in almost every naturally occurring body of water on the planet. Bony fish range in size: • Largest: ocean sunfish (Mola mola), 11 feet, over 5,000 pounds • Smallest: dwarf pygmy goby (Pandaka pygmaea), ½ inch, a fraction of an ounce (This image is life size.) The following guide will help you learn more about the bony fish you can find throughout the New England Aquarium. Much of the guide is keyed to the Giant Ocean Tank, but can be applied to many kinds of fish. Even if you know nothing about fish, you can quickly learn a few things: The shape of a fish’s body, the position of its mouth and the shape of its tail can give you many clues as to its behavior and adaptations. -

Modeling the Evolutionary Loss of Erythroid Genes by Antarctic Icefishes: Analysis of the Hemogen Gene Using Transgenic and Mutant Zebrafish
Modeling the evolutionary loss of erythroid genes by Antarctic icefishes: analysis of the hemogen gene using transgenic and mutant zebrafish by Michael J. Peters B.S. in Biology, University of New Hampshire A dissertation submitted to The Faculty of the College of Science of Northeastern University in partial fulfillment of the requirements for the degree of Doctor of Philosophy June 4, 2018 Dissertation directed by H. William Detrich, III Professor of Marine Molecular Biology and Biochemistry 1 Dedication For my Oma Oswald, who started this journey with me. 2 Acknowledgments I would like to thank my advisor, Dr. H William Detrich III, for encouraging me to be innovative and to pursue cutting-edge research. I thank the members of my committee, Drs. Erin Cram, Rebeca Rosengaus, Steven Vollmer, and Leonard Zon for their many helpful suggestions. I enjoyed working alongside Sandra Parker and Carmen Elenberger and appreciate their support. I also enjoyed working with many students including Caroline Benavides, Carolyn Dubnik, Carmen Elenberger, Laura Goetz, Urjeet Khanwalkar, Ben Moran, Alessia Santilli, Eileen Sheehan, Margaret Streeter, Kathleen Shusdock, and Sierra Smith. I especially thank Jonah Levin, who joined the lab as a high school student and has since continued working with me. I thank Corey Allard and Drs. Donald Yergeau and Jeffrey Grim for collecting samples that I used in my studies. I owe thanks to the staff of the Marine Science Center for their support, including Roberto Valdez, Sonya Simpson, Heather Sears, David Dawson, and Ryan Hill. I thank Drs. Joseph Ayers and Justin Ries for use of their facilities and Drs. -

Respiratory Disorders of Fish
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Disorders of the Respiratory System in Pet and Ornamental Fish a, b Helen E. Roberts, DVM *, Stephen A. Smith, DVM, PhD KEYWORDS Pet fish Ornamental fish Branchitis Gill Wet mount cytology Hypoxia Respiratory disorders Pathology Living in an aquatic environment where oxygen is in less supply and harder to extract than in a terrestrial one, fish have developed a respiratory system that is much more efficient than terrestrial vertebrates. The gills of fish are a unique organ system and serve several functions including respiration, osmoregulation, excretion of nitroge- nous wastes, and acid-base regulation.1 The gills are the primary site of oxygen exchange in fish and are in intimate contact with the aquatic environment. In most cases, the separation between the water and the tissues of the fish is only a few cell layers thick. Gills are a common target for assault by infectious and noninfectious disease processes.2 Nonlethal diagnostic biopsy of the gills can identify pathologic changes, provide samples for bacterial culture/identification/sensitivity testing, aid in fungal element identification, provide samples for viral testing, and provide parasitic organisms for identification.3–6 This diagnostic test is so important that it should be included as part of every diagnostic workup performed on a fish. -
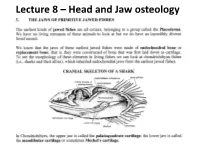
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

Invasive Species of the Pacific Northwest
Invasive Species of the Pacific Northwest: Green Sunfish Lepomis cyanellus Derek Arterburn FISH 423: Olden 12.5.14 Figure 1: Adult Green sunfish Lepomis cyanellus . Photo from http://www.freshwater-fishing- news.com/fish-species-north -america/green-sunfish/ Classification Lepomis cyanellus may have a few teeth, Order: Perciformes which can be found on the tongue. Family: Centrarchidae Additional distinguishing marks are the 7-12 Genus: Lepomis parallel diffused dark bars running ventral to Species: cyanellus dorsal along the side of L. cyanellus, and the bluish-green pattern. The bluish-green Identification coloration takes place on the mainly black/dark brown/olive body, composed of Adult Green Sunfish, Lepomis ctenoid scales, which fades to a lighter cyanellus, commonly reach a total length of ventral color. The dark sides of L. cyanellus 31cm, with juveniles ranging from 12-15cm. are contrast with a yellow/cream ventral Adult Green Sunfish have been known to coloration (Cockerell 1913). The thick reach a maximum weight of one kilogram caudal peduncle is without an adipose fin, (2.2lbs). L. cyanellus is a deep bodied, and the peduncle runs to a rounded, slightly laterally compressed species, with a lateral forked, homocercal caudal fin. The paired line running from the operculum to the fins on Lepomis cyanellus are derived in caudal peduncle. The posterior of the orientation. The Green Sunfish has lateral operculum has a characteristic dark spot placement of the pectoral fins with vertical relatively the same size as the eye, and the insertion, anterior pelvic fins, and spines same size spot may also be found at the base found on the anal and dorsal fins. -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes -

Anatomy and Go Fish! Background
Anatomy and Go Fish! Background Introduction It is important to properly identify fi sh for many reasons: to follow the rules and regulations, for protection against sharp teeth or protruding spines, for the safety of the fi sh, and for consumption or eating purposes. When identifying fi sh, scientists and anglers use specifi c vocabulary to describe external or outside body parts. These body parts are common to most fi sh. The difference in the body parts is what helps distinguish one fi sh from another, while their similarities are used to classify them into groups. There are approximately 29,000 fi sh species in the world. In order to identify each type of fi sh, scientists have grouped them according to their outside body parts, specifi cally the number and location of fi ns, and body shape. Classifi cation Using a system of classifi cation, scientists arrange all organisms into groups based on their similarities. The fi rst system of classifi cation was proposed in 1753 by Carolus Linnaeus. Linnaeus believed that each organism should have a binomial name, genus and species, with species being the smallest organization unit of life. Using Linnaeus’ system as a guide, scientists created a hierarchical system known as taxonomic classifi cation, in which organisms are classifi ed into groups based on their similarities. This hierarchical system moves from largest and most general to smallest and most specifi c: kingdom, phylum, class, order, family, genus, and species. {See Figure 1. Taxonomic Classifi cation Pyramid}. For example, fi sh belong to the kingdom Animalia, the phylum Chordata, and from there are grouped more specifi cally into several classes, orders, families, and thousands of genus and species. -

A Biodiversity Survey of Scavenging Amphipods in a Proposed Marine Protected Area: the Filchner Area in the Weddell Sea, Antarctica
Polar Biology https://doi.org/10.1007/s00300-018-2292-7 ORIGINAL PAPER A biodiversity survey of scavenging amphipods in a proposed marine protected area: the Filchner area in the Weddell Sea, Antarctica Charlotte Havermans1,2 · Meike Anna Seefeldt2,3 · Christoph Held2 Received: 17 October 2017 / Revised: 23 February 2018 / Accepted: 24 February 2018 © Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract An integrative inventory of the amphipod scavenging fauna (Lysianassoidea), combining morphological identifcations with DNA barcoding, is provided here for the Filchner area situated in the south-eastern Weddell Sea. Over 4400 lysianassoids were investigated for species richness and relative abundances, covering 20 diferent stations and using diferent sampling devices, including the southernmost baited traps deployed so far (76°S). High species richness was observed: 29 morphos- pecies of which 5 were new to science. Molecular species delimitation methods were carried out with 109 cytochrome c oxidase I gene (COI) sequences obtained during this study as well as sequences from specimens sampled in other Antarctic regions. These distance-based analyses (trees and the Automatic Barcode Gap Discovery method) indicated the presence of 42 lineages; for 4 species, several (cryptic) lineages were found. More than 96% of the lysianassoids collected with baited traps belonged to the species Orchomenella pinguides s. l. The diversity of the amphipod scavenger guild in this ice-bound ecosystem of the Weddell Sea is discussed in the light of bottom–up selective forces. In this southernmost part of the Weddell Sea, harbouring spawning and nursery grounds for silverfsh and icefshes, abundant fsh and mammalian food falls are likely to represent the major food for scavengers. -

The Opercular Mouth-Opening Mechanism of Largemouth Bass Functions As a 3D Four-Bar Linkage with Three Degrees of Freedom Aaron M
© 2017. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2017) 220, 4612-4623 doi:10.1242/jeb.159079 RESEARCH ARTICLE The opercular mouth-opening mechanism of largemouth bass functions as a 3D four-bar linkage with three degrees of freedom Aaron M. Olsen1,*,‡,§, Ariel L. Camp2,‡ and Elizabeth L. Brainerd2 ABSTRACT (Greene, 1983), the thorax and wings of flying insects (Wootton, The planar, one degree of freedom (1-DoF) four-bar linkage is an 2009), and multiple skeletal elements in the skulls of fishes important model for understanding the function, performance and (Ballintijn, 1969; Westneat, 1990). In addition, four-bar models evolution of numerous biomechanical systems. One such system is have been used to evaluate functional hypotheses by comparing in vivo the opercular mechanism in fishes, which is thought to function like a simulated and kinematics (Westneat, 1991; Van four-bar linkage to depress the lower jaw. While anatomical and Wassenbergh et al., 2005; Roos et al., 2009), to measure how behavioral observations suggest some form of mechanical coupling, force and motion are transmitted through musculoskeletal systems previous attempts to model the opercular mechanism as a planar (Aerts and Verraes, 1984; Adriaens et al., 2001; Van Wassenbergh four-bar have consistently produced poor model fits relative to et al., 2013), and to examine the distribution and evolution of observed kinematics. Using newly developed, open source functional diversity (Westneat, 1995; Alfaro et al., 2004, 2005; mechanism fitting software, we fitted multiple three-dimensional Wainwright et al., 2004; Hulsey and García de León, 2005). (3D) four-bar models with varying DoF to in vivo kinematics in In biomechanical studies, four-bar models have been applied largemouth bass to test whether the opercular mechanism functions most extensively to the skulls of fishes. -

Mitochondrial DNA, Morphology, and the Phylogenetic Relationships of Antarctic Icefishes
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 28 (2003) 87–98 www.elsevier.com/locate/ympev Mitochondrial DNA, morphology, and the phylogenetic relationships of Antarctic icefishes (Notothenioidei: Channichthyidae) Thomas J. Near,a,* James J. Pesavento,b and Chi-Hing C. Chengb a Center for Population Biology, One Shields Avenue, University of California, Davis, CA 95616, USA b Department of Animal Biology, 515 Morrill Hall, University of Illinois, Urbana, IL 61801, USA Received 10 July 2002; revised 4 November 2002 Abstract The Channichthyidae is a lineage of 16 species in the Notothenioidei, a clade of fishes that dominate Antarctic near-shore marine ecosystems with respect to both diversity and biomass. Among four published studies investigating channichthyid phylogeny, no two have produced the same tree topology, and no published study has investigated the degree of phylogenetic incongruence be- tween existing molecular and morphological datasets. In this investigation we present an analysis of channichthyid phylogeny using complete gene sequences from two mitochondrial genes (ND2 and 16S) sampled from all recognized species in the clade. In addition, we have scored all 58 unique morphological characters used in three previous analyses of channichthyid phylogenetic relationships. Data partitions were analyzed separately to assess the amount of phylogenetic resolution provided by each dataset, and phylogenetic incongruence among data partitions was investigated using incongruence length difference (ILD) tests. We utilized a parsimony- based version of the Shimodaira–Hasegawa test to determine if alternative tree topologies are significantly different from trees resulting from maximum parsimony analysis of the combined partition dataset. Our results demonstrate that the greatest phylo- genetic resolution is achieved when all molecular and morphological data partitions are combined into a single maximum parsimony analysis.