Pandemrix Vaccine: Why Was the Public Not Told of Early Warning Signs?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Influenza: Pigs, People & Public Health
National Pork Board | 800-456-7675 | pork.org Public Health Fact Sheet Influenza: Pigs, People & Public Health Authors: Amy L. Vincent, DVM, PhD, USDA-ARS National Animal Disease Center; Marie R. Culhane, DVM, PhD, College of Veterinary Medicine, University of Minnesota; Christopher W. Olsen, DVM PhD, School of Veterinary Medicine and School of Medicine and Public Health, University of Wisconsin-Madison. Reviewers: Jeff B. Bender, DVM MS, University of Minnesota; Andrew Bowman, DVM PhD, The Ohio State University; Todd Davis, PhD, CDC; Ellen Kasari, DVM MS, USDA; Heather Fowler, VMD PhD MPH, National Pork Board Influenza A viruses (IAV) were first isolated from swine in the United States in 1930. Since that time, they remain an economically important cause of respiratory disease in pigs throughout the world and a public health risk. The clinical signs/symptoms of influenza in pigs and people are remarkably similar, with influenza-like illness (ILI) consisting of fever, lethargy, lack of appetite and coughing promi- nent in both species. Influenza viruses can be directly transmitted from pigs to people as “zoonotic” disease agents (pathogens that are transmitted from animals to humans or shared by animals and humans) and cause human infections. Conversely, influenza viruses from people can also infect and cause disease in pigs. These interspecies infections are most likely to occur when people and pigs are in close proximity with one another, such as during livestock exhibits at fairs, live animal markets, swine production barns, and slaughterhouses. Finally, pigs can serve as intermediaries in the generation of novel reassorted influenza viruses since they are also susceptible to infection with avian influenza viruses. -

Vaccination Certificate Information Version: 21 July 2021
معلومات عن شهادة التطعيم، بالعربية 中文疫苗接种凭证信息 Informations sur le certificat de vaccination en FRANÇAIS Информация о сертификате вакцинации на РУССКОМ ЯЗЫКЕ Información sobre el Certificado de Vacunación en ESPAÑOL UN SYSTEM-WIDE COVID-19 VACCINATION PROGRAMME VACCINATION CERTIFICATE INFORMATION VERSION: 21 JULY 2021 ABOUT THE UN SYSTEM-WIDE COVID-19 VACCINATION PROGRAMME The UN is committed to ensuring the protection of its personnel. Tasked by the Secretary-General, the Department of Operational Support (DOS) is leading a coordinated, UN-system-wide effort to ensure the availability of vaccine to UN personnel, their dependents and implementing partners. The roll-out of the UN System-wide COVID-19 Vaccination Programme (the “Programme”) for UN personnel will provide a significant boost to the ability of personnel to stay and deliver on the Organization's mandates, to support beneficiaries in the communities they serve, and to contribute to our on-going work to recover better together from the pandemic. Information about the Programme is available at: https://www.un.org/en/coronavirus/vaccination ABOUT THE VACCINATION CERTIFICATE Upon administration of the requisite vaccine dosage, a certificate of vaccination is generated through the Programme’s registration platform (the “Platform”). The certificate generated by the Platform uses a standardized format, similar to the one used by the WHO in its “International Certificate of Vaccination or Prophylaxis” (Yellow) vaccination booklet. Note: This certificate may not dispense with travel restrictions put in place by the country(ies) of destination. As the nature of the pandemic continues to rapidly evolve, it is highly advisable to check the latest travel regulations. -

Global Dynamics of a Vaccination Model for Infectious Diseases with Asymptomatic Carriers
MATHEMATICAL BIOSCIENCES doi:10.3934/mbe.2016019 AND ENGINEERING Volume 13, Number 4, August 2016 pp. 813{840 GLOBAL DYNAMICS OF A VACCINATION MODEL FOR INFECTIOUS DISEASES WITH ASYMPTOMATIC CARRIERS Martin Luther Mann Manyombe1;2 and Joseph Mbang1;2 Department of Mathematics, Faculty of Science University of Yaounde 1, P.O. Box 812 Yaounde, Cameroon 1;2;3; Jean Lubuma and Berge Tsanou ∗ Department of Mathematics and Applied Mathematics University of Pretoria, Pretoria 0002, South Africa (Communicated by Abba Gumel) Abstract. In this paper, an epidemic model is investigated for infectious dis- eases that can be transmitted through both the infectious individuals and the asymptomatic carriers (i.e., infected individuals who are contagious but do not show any disease symptoms). We propose a dose-structured vaccination model with multiple transmission pathways. Based on the range of the explic- itly computed basic reproduction number, we prove the global stability of the disease-free when this threshold number is less or equal to the unity. Moreover, whenever it is greater than one, the existence of the unique endemic equilibrium is shown and its global stability is established for the case where the changes of displaying the disease symptoms are independent of the vulnerable classes. Further, the model is shown to exhibit a transcritical bifurcation with the unit basic reproduction number being the bifurcation parameter. The impacts of the asymptomatic carriers and the effectiveness of vaccination on the disease transmission are discussed through through the local and the global sensitivity analyses of the basic reproduction number. Finally, a case study of hepatitis B virus disease (HBV) is considered, with the numerical simulations presented to support the analytical results. -

Antiviral Agents Active Against Influenza a Viruses
REVIEWS Antiviral agents active against influenza A viruses Erik De Clercq Abstract | The recent outbreaks of avian influenza A (H5N1) virus, its expanding geographic distribution and its ability to transfer to humans and cause severe infection have raised serious concerns about the measures available to control an avian or human pandemic of influenza A. In anticipation of such a pandemic, several preventive and therapeutic strategies have been proposed, including the stockpiling of antiviral drugs, in particular the neuraminidase inhibitors oseltamivir (Tamiflu; Roche) and zanamivir (Relenza; GlaxoSmithKline). This article reviews agents that have been shown to have activity against influenza A viruses and discusses their therapeutic potential, and also describes emerging strategies for targeting these viruses. HXNY In the face of the persistent threat of human influenza A into the interior of the virus particles (virions) within In the naming system for (H3N2, H1N1) and B infections, the outbreaks of avian endosomes, a process that is needed for the uncoating virus strains, H refers to influenza (H5N1) in Southeast Asia, and the potential of to occur. The H+ ions are imported through the M2 haemagglutinin and N a new human or avian influenza A variant to unleash a (matrix 2) channels10; the transmembrane domain of to neuraminidase. pandemic, there is much concern about the shortage in the M2 protein, with the amino-acid residues facing both the number and supply of effective anti-influenza- the ion-conducting pore, is shown in FIG. 3a (REF. 11). virus agents1–4. There are, in principle, two mechanisms Amantadine has been postulated to block the interior by which pandemic influenza could originate: first, by channel within the tetrameric M2 helix bundle12. -

Recent Advances of Vaccine Adjuvants for Infectious Diseases
http://dx.doi.org/10.4110/in.2015.15.2.51 REVIEW ARTICLE pISSN 1598-2629 eISSN 2092-6685 Recent Advances of Vaccine Adjuvants for Infectious Diseases Sujin Lee1,2* and Minh Trang Nguyen1,2 1Department of Pediatrics, Emory University, School of Medicine, 2Children's Healthcare of Atlanta, Atlanta, Georgia 30322, USA Vaccines are the most effective and cost-efficient method for VACCINES preventing diseases caused by infectious pathogens. Despite the great success of vaccines, development of safe Infectious diseases remain the second leading cause of death and strong vaccines is still required for emerging new patho- worldwide after cardiovascular disease, but the leading cause gens, re-emerging old pathogens, and in order to improve the of death in infants and children (1). Vaccination is the most inadequate protection conferred by existing vaccines. One of efficient tool for preventing a variety of infectious diseases. the most important strategies for the development of effec- The ultimate goal of vaccination is to generate a patho- tive new vaccines is the selection and usage of a suitable adjuvant. Immunologic adjuvants are essential for enhancing gen-specific immune response providing long-lasting pro- vaccine potency by improvement of the humoral and/or tection against infection (2). Despite the significant success cell-mediated immune response to vaccine antigens. Thus, of vaccines, development of safe and strong vaccines is still formulation of vaccines with appropriate adjuvants is an at- required due to the emergence of new pathogens, re-emer- tractive approach towards eliciting protective and long-last- gence of old pathogens and suboptimal protection conferred ing immunity in humans. -

Considerations for Causality Assessment of Neurological And
Occasional essay J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp-2021-326924 on 6 August 2021. Downloaded from Considerations for causality assessment of neurological and neuropsychiatric complications of SARS- CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder Matt Butler ,1 Arina Tamborska,2,3 Greta K Wood,2,3 Mark Ellul,4 Rhys H Thomas,5,6 Ian Galea ,7 Sarah Pett,8 Bhagteshwar Singh,3 Tom Solomon,4 Thomas Arthur Pollak,9 Benedict D Michael,2,3 Timothy R Nicholson10 For numbered affiliations see INTRODUCTION More severe potential adverse effects in the open- end of article. The scientific community rapidly responded to label phase of vaccine roll- outs are being collected the COVID-19 pandemic by developing novel through national surveillance systems. In the USA, Correspondence to SARS- CoV-2 vaccines (table 1). As of early June Dr Timothy R Nicholson, King’s roughly 372 adverse events have been reported per College London, London WC2R 2021, an estimated 2 billion doses have been million doses, which is a lower rate than expected 1 2LS, UK; timothy. nicholson@ administered worldwide. Neurological adverse based on the clinical trials.6 kcl. ac. uk events following immunisation (AEFI), such as In the UK, adverse events are reported via the cerebral venous sinus thrombosis and demyelin- MB and AT are joint first Coronavirus Yellow Card reporting website. As of ating episodes, have been reported. In some coun- authors. early June 2021, approximately 250 000 Yellow tries, these have led to the temporary halting of BDM and TRN are joint senior Cards have been submitted, equating to around authors. -

Questions & Answers on Influenza
101 Questions & Answers on Influenza101 101 Questions & Answers Prof. Dr. A.D.M.E. (Ab) Osterhaus is David De Pooter is working at Link Inc on professor of virology at Erasmus Medical since 2003, the Antwerp (Belgium) based Centre Rotterdam, and professor of communication consultancy agency, Environmental Virology at the Utrecht specialised in strategic communication University. Fascinated by the ingenious and social marketing. Link Inc is working ways viruses circumvent the immune with the European Scientific working Influenza system of their hosts to multiply and Group on Influenza (ESWI) since 1998 and spread, Osterhaus started his quest at the is taking care of the positioning of the interface of virology and immunology. He group, the strategy and the implementa Ab Osterhaus quickly translated new insights in this tion of the strategy by developing complex field to applications in animal and targeted communication tools. In this human vaccinology. In addition, he started capacity, David De Pooter is a professional David De Pooter his work on virus discovery, not only writer on medical topics and a communi focussing on the identification of a series cation manager of ESWI. As such he has of animal viruses, but also of new human established a fruitful and long standing viruses. collaboration with Prof Ab Osterhaus. (www.linkinc.be) 101 Questions & Answers on Influenza 101 101 Questions & Answers on Influenza Ab Osterhaus David De Pooter Elsevier, Maarssen © Elsevier, Maarssen 2009 Design: Studio Bassa, Culemborg Elsevier is an imprint of Reed Business bv, PO Box 1110, 3600 BC Maarssen, The Netherlands. To order: Elsevier Gezondheidszorg, Marketing dept., Antwoordnummer 2594 (freepost), 3600 VB Maarssen, The Netherlands. -

Vaccine Hesitancy
WHY CHILDREN WORKSHOP ON IMMUNIZATIONS ARE NOT VACCINATED? VACCINE HESITANCY José Esparza MD, PhD - Adjunct Professor, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD, USA - Robert Koch Fellow, Robert Koch Institute, Berlin, Germany - Senior Advisor, Global Virus Network, Baltimore, MD, USA. Formerly: - Bill & Melinda Gates Foundation, Seattle, WA, USA - World Health Organization, Geneva, Switzerland The value of vaccination “The impact of vaccination on the health of the world’s people is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth” Stanley Plotkin (2013) VACCINES VAILABLE TO PROTECT AGAINST MORE DISEASES (US) BASIC VACCINES RECOMMENDED BY WHO For all: BCG, hepatitis B, polio, DTP, Hib, Pneumococcal (conjugated), rotavirus, measles, rubella, HPV. For certain regions: Japanese encephalitis, yellow fever, tick-borne encephalitis. For some high-risk populations: typhoid, cholera, meningococcal, hepatitis A, rabies. For certain immunization programs: mumps, influenza Vaccines save millions of lives annually, worldwide WHAT THE WORLD HAS ACHIEVED: 40 YEARS OF INCREASING REACH OF BASIC VACCINES “Bill Gates Chart” 17 M GAVI 5.6 M 4.2 M Today (ca 2015): <5% of children in GAVI countries fully immunised with the 11 WHO- recommended vaccines Seth Berkley (GAVI) The goal: 50% of children in GAVI countries fully immunised by 2020 Seth Berkley (GAVI) The current world immunization efforts are achieving: • Equity between high and low-income countries • Bringing the power of vaccines to even the world’s poorest countries • Reducing morbidity and mortality in developing countries • Eliminating and eradicating disease WHY CHILDREN ARE NOT VACCINATED? •Vaccines are not available •Deficient health care systems •Poverty •Vaccine hesitancy (reticencia a la vacunacion) VACCINE HESITANCE: WHO DEFINITION “Vaccine hesitancy refers to delay in acceptance or refusal of vaccines despite availability of vaccination services. -
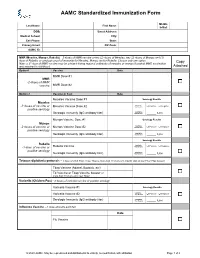
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

AJIC-Oseltamivir-Stockpile-Paper.Pdf
American Journal of Infection Control 45 (2017) 303-5 Contents lists available at ScienceDirect American Journal of Infection Control American Journal of Infection Control journal homepage: www.ajicjournal.org Brief Report Oseltamivir for pandemic influenza preparation: Maximizing the use of an existing stockpile Twisha S. Patel PharmD a, Sandro Cinti MD b, Duxin Sun PhD c, Siwei Li PhD c, Ruijuan Luo PhD c, Bo Wen PhD c, Brian A. Gallagher JD d,e, James G. Stevenson PharmD a,c,* a Pharmacy Services, University of Michigan Health System, Ann Arbor, MI b Infectious Diseases, University of Michigan Health System, Ann Arbor, MI c University of Michigan College of Pharmacy, Ann Arbor, MI d Marshall University School of Pharmacy, Huntington, WV e Joan C. Edwards School of Medicine, Huntington, WV With the threat of significant morbidity and mortality following an influenza pandemic, stockpiling of antiviral agents such as oseltamivir is recommended. Shelf-life extension was explored to maximize use of an existing stockpile. This analysis demonstrated that oseltamivir retains potency defined by United States Pharmacopeia acceptance criteria beyond the labeled expiration date. © 2017 Association for Professionals in Infection Control and Epidemiology, Inc. Published by Elsevier Inc. All rights reserved. BACKGROUND hospital employees because up to 35% are expected to become ill even with the institution of optimal infection control practices during Infection with the influenza virus can lead to devastating com- an influenza pandemic.7 Although imperative, this strategy created plications, including mortality.1 The influenza pandemic in 1918 and asignificantfinancialburdenonourinstitution:A5-daytreat- the Asian H5N1 outbreak in 2005 taught our nation a valuable lesson ment course of oseltamivir is considerably expensive ($120/box of about the importance of creating a national preparedness plan to 10 75-mg capsules, University of Michigan Health System, unpub- combat the spread of infection. -

1 Title: Interim Report of a Phase 2 Randomized Trial of a Plant
medRxiv preprint doi: https://doi.org/10.1101/2021.05.14.21257248; this version posted May 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. 1 Title: Interim Report of a Phase 2 Randomized Trial of a Plant-Produced Virus-Like Particle 2 Vaccine for Covid-19 in Healthy Adults Aged 18-64 and Older Adults Aged 65 and Older 3 Authors: Philipe Gobeil1, Stéphane Pillet1, Annie Séguin1, Iohann Boulay1, Asif Mahmood1, 4 Donald C Vinh 2, Nathalie Charland1, Philippe Boutet3, François Roman3, Robbert Van Der 5 Most4, Maria de los Angeles Ceregido Perez3, Brian J Ward1,2†, Nathalie Landry1† 6 Affiliations: 1 Medicago Inc., 1020 route de l’Église office 600, Québec, QC, Canada, G1V 7 3V9; 2 Research Institute of the McGill University Health Centre, 1001 Decarie St, Montreal, 8 QC H4A 3J1; 3 GlaxoSmithKline Biologicals SA (Vaccines), Avenue Fleming 20, 1300 Wavre, 9 Belgium; 4 GlaxoSmithKline Biologicals SA (Vaccines), rue de l’Institut 89, 1330 Rixensart, 10 Belgium; † These individuals are equally credited as senior authors. 11 * Corresponding author: Nathalie Landry, 1020 Route de l’Église, Bureau 600, Québec, Qc, 12 Canada, G1V 3V9; Tel. 418 658 9393; Fax. 418 658 6699; [email protected] 13 Abstract 14 The rapid spread of SARS-CoV-2 globally continues to impact humanity on a global scale with 15 rising morbidity and mortality. Despite the development of multiple effective vaccines, new 16 vaccines continue to be required to supply ongoing demand. -

Evidence Assessment STRATEGIC ADVISORY GROUP of EXPERTS (SAGE) on IMMUNIZATION Meeting 15 March 2021
Evidence Assessment STRATEGIC ADVISORY GROUP OF EXPERTS (SAGE) ON IMMUNIZATION Meeting 15 March 2021 FOR RECOMMENDATION BY SAGE Prepared by the SAGE Working Group on COVID-19 vaccines EVIDENCE ASSESSMENT: Ad26.COV2.S COVID-19 vaccine Key evidence to inform policy recommendations on the use of Ad26.COV2.S COVID-19 vaccine Evidence retrieval • Based on WHO and Cochrane living mapping and living systematic review of Covid-19 trials (www.covid-nma.com/vaccines) Retrieved evidence Majority of data considered for policy recommendations on Ad26.COV2.S Covid-19 vaccine are published in scientific peer reviewed journals: • Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. NEJM. February 2021. DOI: 10.1056/NEJMoa2034201. Jerald Sadoff, Mathieu Le Gars, Georgi Shukarev, et al. • FDA briefing document Janssen Ad26.COV2.S (COVID-19) Vaccine • Ad26 platform literature: Custers et al. Vaccines based on the Ad26 platform; Vaccine 2021 Quality assessment Type of bias Randomization Deviations from Missing outcome Measurement of Selection of the Overall risk of intervention data the outcome reported results bias Working Group Low Low Low Low Low LOW judgment 1 EVIDENCE ASSESSMENT The SAGE Working Group specifically considered the following issues: 1. Trial endpoints used in comparison with other vaccines Saad Omer 2. Evidence on vaccine efficacy in the total population and in different subgroups Cristiana Toscano 3. Evidence of vaccine efficacy against asymptomatic infections, reducing severity, hospitalisations, deaths, and efficacy against virus variants of concern Annelies Wilder-Smith 4. Vaccination after SARS-CoV2 infection. 5. Evidence on vaccine safety in the total population and in different subgroups Sonali Kochhar 6.