Detection and Molecular Characterization of Mogiana Tick Virus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TICKS in RELATION to HUMAN DISEASES CAUSED by <I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Navy Research U.S. Department of Defense 1967 TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES Harry Hoogstraal Follow this and additional works at: https://digitalcommons.unl.edu/usnavyresearch This Article is brought to you for free and open access by the U.S. Department of Defense at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in U.S. Navy Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES1,2 By HARRY HOOGSTRAAL Department oj Medical Zoology, United States Naval Medical Research Unit Number Three, Cairo, Egypt, U.A.R. Rickettsiae (185) are obligate intracellular parasites that multiply by binary fission in the cells of both vertebrate and invertebrate hosts. They are pleomorphic coccobacillary bodies with complex cell walls containing muramic acid, and internal structures composed of ribonucleic and deoxyri bonucleic acids. Rickettsiae show independent metabolic activity with amino acids and intermediate carbohydrates as substrates, and are very susceptible to tetracyclines as well as to other antibiotics. They may be considered as fastidious bacteria whose major unique character is their obligate intracellu lar life, although there is at least one exception to this. In appearance, they range from coccoid forms 0.3 J.I. in diameter to long chains of bacillary forms. They are thus intermediate in size between most bacteria and filterable viruses, and form the family Rickettsiaceae Pinkerton. They stain poorly by Gram's method but well by the procedures of Macchiavello, Gimenez, and Giemsa. -

Ehrlichiosis and Anaplasmosis Are Tick-Borne Diseases Caused by Obligate Anaplasmosis: Intracellular Bacteria in the Genera Ehrlichia and Anaplasma
Ehrlichiosis and Importance Ehrlichiosis and anaplasmosis are tick-borne diseases caused by obligate Anaplasmosis: intracellular bacteria in the genera Ehrlichia and Anaplasma. These organisms are widespread in nature; the reservoir hosts include numerous wild animals, as well as Zoonotic Species some domesticated species. For many years, Ehrlichia and Anaplasma species have been known to cause illness in pets and livestock. The consequences of exposure vary Canine Monocytic Ehrlichiosis, from asymptomatic infections to severe, potentially fatal illness. Some organisms Canine Hemorrhagic Fever, have also been recognized as human pathogens since the 1980s and 1990s. Tropical Canine Pancytopenia, Etiology Tracker Dog Disease, Ehrlichiosis and anaplasmosis are caused by members of the genera Ehrlichia Canine Tick Typhus, and Anaplasma, respectively. Both genera contain small, pleomorphic, Gram negative, Nairobi Bleeding Disorder, obligate intracellular organisms, and belong to the family Anaplasmataceae, order Canine Granulocytic Ehrlichiosis, Rickettsiales. They are classified as α-proteobacteria. A number of Ehrlichia and Canine Granulocytic Anaplasmosis, Anaplasma species affect animals. A limited number of these organisms have also Equine Granulocytic Ehrlichiosis, been identified in people. Equine Granulocytic Anaplasmosis, Recent changes in taxonomy can make the nomenclature of the Anaplasmataceae Tick-borne Fever, and their diseases somewhat confusing. At one time, ehrlichiosis was a group of Pasture Fever, diseases caused by organisms that mostly replicated in membrane-bound cytoplasmic Human Monocytic Ehrlichiosis, vacuoles of leukocytes, and belonged to the genus Ehrlichia, tribe Ehrlichieae and Human Granulocytic Anaplasmosis, family Rickettsiaceae. The names of the diseases were often based on the host Human Granulocytic Ehrlichiosis, species, together with type of leukocyte most often infected. -

An Insight Into the Ecobiology, Vector Significance and Control of Hyalomma Ticks (Acari: Ixodidae): a Review
Accepted Manuscript Title: AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW Authors: M.S. Sajid, A. Kausar, A. Iqbal, H. Abbas, Z. Iqbal, M.K. Jones PII: S0001-706X(18)30862-3 DOI: https://doi.org/10.1016/j.actatropica.2018.08.016 Reference: ACTROP 4752 To appear in: Acta Tropica Received date: 6-7-2018 Revised date: 10-8-2018 Accepted date: 12-8-2018 Please cite this article as: Sajid MS, Kausar A, Iqbal A, Abbas H, Iqbal Z, Jones MK, AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW, Acta Tropica (2018), https://doi.org/10.1016/j.actatropica.2018.08.016 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW M. S. SAJID 1 2 *, A. KAUSAR 3, A. IQBAL 4, H. ABBAS 5, Z. IQBAL 1, M. K. JONES 6 1. Department of Parasitology, Faculty of Veterinary Science, University of Agriculture, Faisalabad-38040, Pakistan. 2. One Health Laboratory, Center for Advanced Studies in Agriculture and Food Security (CAS-AFS) University of Agriculture, Faisalabad-38040, Pakistan. -

Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Spring 2009 Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences Erica Janelle Burkman Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Recommended Citation Burkman, Erica Janelle, "Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences" (2009). Electronic Theses and Dissertations. 704. https://digitalcommons.georgiasouthern.edu/etd/704 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. GENETIC STRUCTURE OF AMBLYOMMA CAJENNENSE (ACARI: IXODIDAE) POPULATIONS BASED ON MITOCHONDRIAL GENE SEQUENCES by ERICA JANELLE BURKMAN (Under the Direction of Dr. Lorenza Beati) ABSTRACT Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) is a common tick species that has a large geographic distribution from the southern regions of the United States (Texas), to the Caribbean Islands, Central, and South America. This tick is a vector of the agent of Brazilian spotted fever, an often fatal disease in South America. Throughout its geographic range, populations of A. cajennense have shown differences in ecological adaptation while feeding on a variety of hosts ranging from livestock, birds, and humans. In order to examine the taxonomic status and phylogeographic evolution of this species, we analyzed mitochondrial 12S rDNA, control region (d-loop), and cytochrome oxidase II gene sequences of A. -

Morphometrics of Amblyomma Mixtum in the State of Veracruz, Mexico
pathogens Article Morphometrics of Amblyomma mixtum in the State of Veracruz, Mexico Mariel Aguilar-Domínguez 1, Dora Romero-Salas 1,* , Sokani Sánchez-Montes 2, Ricardo Serna-Lagunes 3 , Greta Rosas-Saito 4, Anabel Cruz-Romero 1 and Adalberto A. Pérez de León 5,6 1 Laboratorio de Parasitología, rancho “Torreón del Molino”, Facultad de Medicina Veterinaria y Zootecnia, Universidad Veracruzana, Veracruz 91697, Mexico; [email protected] (M.A.-D.); [email protected] (A.C.-R.) 2 Facultad de Ciencias Biológicas y Agropecuarias Región Tuxpan, Universidad Veracruzana, Tuxpam 92870, Mexico; [email protected] 3 Laboratorio de Bioinformática y Bioestadística, Facultad de Ciencias Biológicas y Agropecuarias, Universidad Veracruzana, Córdoba 94945, Mexico; [email protected] 4 Red de Estudios Moleculares Avanzados, Instituto de Ecología, Xalapa 91073, Mexico; [email protected] 5 USDA-ARS Knipling-Bushland U.S. Veterinary Pest Genomics Center and Livestock Insects Research Laboratory, Kerrville, TX 78028, USA; [email protected] 6 USDA-ARS San Joaquin Valley Agricultural Sciences Center, Parlier, CA 93648, USA * Correspondence: [email protected]; Tel.: +52-(229)-9342075 Abstract: The tick Amblyomma mixtum is an ectoparasite of veterinary and public health importance because of its role as a vector of zoonotic pathogens. However, little is known about A. mixtum intraspecific variability and if morphological differentiation exists between populations across its geographic range. This study aimed to determine by electron microscopy the morphological vari- ability of A. mixtum populations in the state of Veracruz, which has a large livestock population Citation: Aguilar-Domínguez, M.; among states in Mexico. Forty male and 40 female A. mixtum collected from the 10 natural regions Romero-Salas, D.; Sánchez-Montes, S.; of Veracruz state were analyzed microscopically to accomplish main component analysis for each Serna-Lagunes, R.; Rosas-Saito, G.; sex. -

A Look Into Bunyavirales Genomes: Functions of Non-Structural (NS) Proteins
viruses Review A Look into Bunyavirales Genomes: Functions of Non-Structural (NS) Proteins Shanna S. Leventhal, Drew Wilson, Heinz Feldmann and David W. Hawman * Laboratory of Virology, Rocky Mountain Laboratories, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT 59840, USA; [email protected] (S.S.L.); [email protected] (D.W.); [email protected] (H.F.) * Correspondence: [email protected]; Tel.: +1-406-802-6120 Abstract: In 2016, the Bunyavirales order was established by the International Committee on Taxon- omy of Viruses (ICTV) to incorporate the increasing number of related viruses across 13 viral families. While diverse, four of the families (Peribunyaviridae, Nairoviridae, Hantaviridae, and Phenuiviridae) contain known human pathogens and share a similar tri-segmented, negative-sense RNA genomic organization. In addition to the nucleoprotein and envelope glycoproteins encoded by the small and medium segments, respectively, many of the viruses in these families also encode for non-structural (NS) NSs and NSm proteins. The NSs of Phenuiviridae is the most extensively studied as a host interferon antagonist, functioning through a variety of mechanisms seen throughout the other three families. In addition, functions impacting cellular apoptosis, chromatin organization, and transcrip- tional activities, to name a few, are possessed by NSs across the families. Peribunyaviridae, Nairoviridae, and Phenuiviridae also encode an NSm, although less extensively studied than NSs, that has roles in antagonizing immune responses, promoting viral assembly and infectivity, and even maintenance of infection in host mosquito vectors. Overall, the similar and divergent roles of NS proteins of these Citation: Leventhal, S.S.; Wilson, D.; human pathogenic Bunyavirales are of particular interest in understanding disease progression, viral Feldmann, H.; Hawman, D.W. -
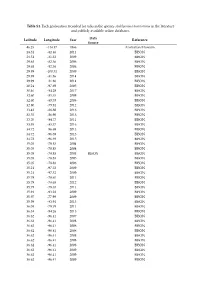
Table S1. Each Geolocation Recorded for Ticks in the Species Amblyomma Americanum in the Literature and Publicly Available Online Databases
Table S1. Each geolocation recorded for ticks in the species Amblyomma americanum in the literature and publicly available online databases. Data Latitude Longitude Year Reference Source 46.25 -114.17 1966 Australian Museum 28.31 -82.46 2011 BISON 28.51 -81.32 2009 BISON 29.68 -82.36 2006 BISON 29.68 -82.36 2006 BISON 29.99 -100.31 2009 BISON 29.99 -81.86 2014 BISON 29.99 -81.86 2014 BISON 30.24 -97.69 2005 BISON 30.46 -84.28 2017 BISON 32.60 -85.35 2008 BISON 32.60 -85.35 2008 BISON 32.80 -79.94 2012 BISON 33.42 -88.88 2016 BISON 33.55 -86.90 2013 BISON 33.70 -84.77 2011 BISON 33.95 -83.37 2016 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2015 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON BISON 35.05 -78.83 2005 BISON 35.05 -78.83 2006 BISON 35.21 -97.32 2009 BISON 35.21 -97.32 2009 BISON 35.79 -78.65 2011 BISON 35.79 -78.65 2012 BISON 35.79 -78.65 2011 BISON 35.91 -93.22 2009 BISON 35.97 -77.99 2009 BISON 35.99 -83.94 2015 BISON 36.08 -79.79 2011 BISON 36.34 -94.26 2015 BISON 36.62 -96.41 2007 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2011 BISON 36.62 -96.41 2012 BISON 36.62 -96.41 2012 BISON 37.36 -77.05 2014 BISON 37.62 -84.87 2016 BISON 37.83 -78.28 2011 BISON 37.97 -85.70 2016 BISON 37.97 -85.70 2016 BISON 38.22 -75.31 2014 BISON 38.43 -88.43 -

Rocky Mountain Spotted Fever
Zoonosis Update Rocky Mountain spotted fever Ronald D. Warner, DVM, MPVM, PhD, DACVPM, and Wallace W. Marsh, MD, FAAP ocky Mountain spotted fever (RMSF), a classic SFG rickettsiae are transmitted by arthropods and cause Rmetazoonosis that involves both vertebrate and non- various illnesses worldwide, R rickettsii is the only one vertebrate reservoir hosts, is a seasonal disease of dogs known to be pathogenic for both animals and humans and humans in the Americas. The clinical illness was in the Western Hemisphere. first described among Native Americans, soldiers, and settlers in the Bitterroot River and Snake River valleys of Cycle of the Organism in Nature Montana and Idaho during the late 1890s, but remained and the Vectors unrecognized in dogs until the late 1970s. The causative Rickettsia rickettsii are maintained in nature by organism, Rickettsia rickettsii, was first described by transstadial passage within, and transovarial (vertical) Howard T. Ricketts in 1909 and is maintained in nature transmission between, generations of ixodid ticks. These by ixodid (hard-bodied) ticks via transmission to-and- ticks also vector R rickettsii to and from various rodent from various rodent reservoirs. As primary reservoir reservoirs and other small mammals. Naïve larval and hosts, the ticks vector R rickettsii to larger mammals; nymphal ticks become infected while feeding on small however, dogs and humans are the only ones that dis- rodents (eg, mice, voles, squirrels, or chipmunks) with play clinically recognizable illnesses. Rickettsia rickettsii acute rickettsemia.2,5 To enable transovarial transmission, are not naturally transmitted dog-to-dog, dog-to- female ticks need to ingest numerous rickettsiae or be human, or human-to-human. -

Sustained RNA Virome Diversity in Antarctic Penguins and Their Ticks
The ISME Journal (2020) 14:1768–1782 https://doi.org/10.1038/s41396-020-0643-1 ARTICLE Sustained RNA virome diversity in Antarctic penguins and their ticks 1 2 2 3 2 1 Michelle Wille ● Erin Harvey ● Mang Shi ● Daniel Gonzalez-Acuña ● Edward C. Holmes ● Aeron C. Hurt Received: 11 December 2019 / Revised: 16 March 2020 / Accepted: 20 March 2020 / Published online: 14 April 2020 © The Author(s) 2020. This article is published with open access Abstract Despite its isolation and extreme climate, Antarctica is home to diverse fauna and associated microorganisms. It has been proposed that the most iconic Antarctic animal, the penguin, experiences low pathogen pressure, accounting for their disease susceptibility in foreign environments. There is, however, a limited understanding of virome diversity in Antarctic species, the extent of in situ virus evolution, or how it relates to that in other geographic regions. To assess whether penguins have limited microbial diversity we determined the RNA viromes of three species of penguins and their ticks sampled on the Antarctic peninsula. Using total RNA sequencing we identified 107 viral species, comprising likely penguin associated viruses (n = 13), penguin diet and microbiome associated viruses (n = 82), and tick viruses (n = 8), two of which may have the potential to infect penguins. Notably, the level of virome diversity revealed in penguins is comparable to that seen in Australian waterbirds, including many of the same viral families. These data run counter to the idea that penguins are subject 1234567890();,: 1234567890();,: to lower pathogen pressure. The repeated detection of specific viruses in Antarctic penguins also suggests that rather than being simply spill-over hosts, these animals may act as key virus reservoirs. -

Analyzing the Potential Transmission of Tick-Borne Pathogens to Man
Revista da Sociedade Brasileira de Medicina Tropical ARTIGO 32(6):613-619, nov-dez, 1999. Report on ticks collected in the Southeast and Mid-West regions of Brazil: analyzing the potential transmission of tick-borne pathogens to man Relato sobre carrapatos coletados no Sudoeste e no Centro-oeste do Brasil analisando a potencial transmissão de microorganismos de carrapatos para o homem Luiz Tadeu Moraes Figueiredo1, Soraya Jabur Badra1, Luiz Eloy Pereira2 and Matias Pablo Juan Szabó3 Abstract Specimens of ticks were collected in 1993, 1996, 1997, and 1998, mostly from wild and domestic animals in the Southeast and Mid-West regions of Brazil. Nine species of Amblyommidae were identified: Anocentor nitens, Amblyomma cajennense, Amblyomma ovale, Amblyomma fulvum, Amblyomma striatum, Amblyomma rotundatum, Boophilus microplus, Boophilus annulatus, and Rhipicephalus sanguineus. The potential of these tick species as transmitters of pathogens to man was analyzed. A Flaviviridade Flavivirus was isolated from Amblyomma cajennense specimens collected from a sick capybara (Hydrochaeris hydrochaeris). Amblyomma cajennense is the main transmitter of Rickettsia rickettsii (=R. rickettsi), the causative agent of spotted fever in Brazil. Wild mammals, mainly capybaras and deer, infested by ticks and living in close contact with cattle, horses and dogs, offer the risk of transmission of wild zoonosis to these domestic animals and to man. Key-words: Brazilian ticks. Tick-borne pathogens. Resumo Foram coletados espécimes de carrapatos em 1993, 1996, 1997, e 1998, principalmente de animais selvagens e domésticos, nas Regiões Sudeste e Centro-oeste do Brasil. Nove espécies de Amblyommidae foram identificadas: Anocentor Nitens, Amblyomma cajennense, Amblyomma ovale, Amblyomma fulvum, Amblyomma striatum, Amblyomma rotundatum, Boophilus microplus, Boophilus annulatus e Rhipicephalus sanguineus. -

First Evidence of Ehrlichia Minasensis Infection in Horses from Brazil
pathogens Article First Evidence of Ehrlichia minasensis Infection in Horses from Brazil Lívia S. Muraro 1, Aneliza de O. Souza 2, Tamyres N. S. Leite 2, Stefhano L. Cândido 3, Andréia L. T. Melo 4, Hugo S. Toma 5 , Mariana B. Carvalho 4, Valéria Dutra 3, Luciano Nakazato 3, Alejandro Cabezas-Cruz 6 and Daniel M. de Aguiar 1,* 1 Laboratory of Virology and Rickettsial Infections, Veterinary Hospital, Federal University of Mato Grosso (UFMT), Av. Fernando Correa da Costa 2367, Cuiabá 78090-900, Brazil; [email protected] 2 Veterinary Clinical Laboratory, Department of Veterinary Clinics, University of Cuiabá (UNIC), Av. Manoel José de Arruda 3100, Cuiabá 78065-900, Brazil; [email protected] (A.d.O.S.); [email protected] (T.N.S.L.) 3 Laboratory of Microbiology and Molecular Biology, Veterinary Hospital of the Faculty of Veterinary Medicine, Federal University of Mato Grosso (UFMT), Av. Fernando Correa da Costa 2367, Cuiabá 78090-900, Brazil; [email protected] (S.L.C.); [email protected] (V.D.); [email protected] (L.N.) 4 Veterinary of Clinical, Veterinary Medicine College, University of Cuiabá (UNIC), Av. Manoel José de Arruda 3100, Cuiabá 78065-900, Brazil; [email protected] (A.L.T.M.); [email protected] (M.B.C.) 5 Veterinary Medicine Department, Federal University of Lavras (UFLA), Campus Universitário, Mailbox 3037, Lavras 37200-000, Brazil; hugo.toma@ufla.br 6 Anses, INRAE, Ecole Nationale Vétérinaire d’Alfort, UMR BIPAR, Laboratoire de Santé Animale, F-94700 Maisons-Alfort, France; [email protected] * Correspondence: [email protected] Citation: Muraro, L.S.; Souza, A.d.O.; Leite, T.N.S.; Cândido, S.L.; Melo, Abstract: The genus Ehrlichia includes tick-borne bacterial pathogens affecting humans, domestic and A.L.T.; Toma, H.S.; Carvalho, M.B.; wild mammals. -

Bat-Borne Virus Diversity, Spillover and Emergence
REVIEWS Bat-borne virus diversity, spillover and emergence Michael Letko1,2 ✉ , Stephanie N. Seifert1, Kevin J. Olival 3, Raina K. Plowright 4 and Vincent J. Munster 1 ✉ Abstract | Most viral pathogens in humans have animal origins and arose through cross-species transmission. Over the past 50 years, several viruses, including Ebola virus, Marburg virus, Nipah virus, Hendra virus, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory coronavirus (MERS-CoV) and SARS-CoV-2, have been linked back to various bat species. Despite decades of research into bats and the pathogens they carry, the fields of bat virus ecology and molecular biology are still nascent, with many questions largely unexplored, thus hindering our ability to anticipate and prepare for the next viral outbreak. In this Review, we discuss the latest advancements and understanding of bat-borne viruses, reflecting on current knowledge gaps and outlining the potential routes for future research as well as for outbreak response and prevention efforts. Bats are the second most diverse mammalian order on as most of these sequences span polymerases and not Earth after rodents, comprising approximately 22% of all the surface proteins that often govern cellular entry, little named mammal species, and are resident on every conti- progress has been made towards translating sequence nent except Antarctica1. Bats have been identified as nat- data from novel viruses into a risk-based assessment ural reservoir hosts for several emerging viruses that can to quantify zoonotic potential and elicit public health induce severe disease in humans, including RNA viruses action. Further hampering this effort is an incomplete such as Marburg virus, Hendra virus, Sosuga virus and understanding of the animals themselves, their distribu- Nipah virus.