Modeling Potential Habitat for Amblyomma Tick Species in California
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TICKS in RELATION to HUMAN DISEASES CAUSED by <I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Navy Research U.S. Department of Defense 1967 TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES Harry Hoogstraal Follow this and additional works at: https://digitalcommons.unl.edu/usnavyresearch This Article is brought to you for free and open access by the U.S. Department of Defense at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in U.S. Navy Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES1,2 By HARRY HOOGSTRAAL Department oj Medical Zoology, United States Naval Medical Research Unit Number Three, Cairo, Egypt, U.A.R. Rickettsiae (185) are obligate intracellular parasites that multiply by binary fission in the cells of both vertebrate and invertebrate hosts. They are pleomorphic coccobacillary bodies with complex cell walls containing muramic acid, and internal structures composed of ribonucleic and deoxyri bonucleic acids. Rickettsiae show independent metabolic activity with amino acids and intermediate carbohydrates as substrates, and are very susceptible to tetracyclines as well as to other antibiotics. They may be considered as fastidious bacteria whose major unique character is their obligate intracellu lar life, although there is at least one exception to this. In appearance, they range from coccoid forms 0.3 J.I. in diameter to long chains of bacillary forms. They are thus intermediate in size between most bacteria and filterable viruses, and form the family Rickettsiaceae Pinkerton. They stain poorly by Gram's method but well by the procedures of Macchiavello, Gimenez, and Giemsa. -

Comparative Analysis of Germ Cells and DNA of the Genus Amblyomma: Adding New Data on Amblyomma Maculatum and Amblyomma Ovale Species (Acari: Ixodidae)
Parasitol Res (2017) 116:2883–2892 DOI 10.1007/s00436-017-5592-x ORIGINAL PAPER Comparative analysis of germ cells and DNA of the genus Amblyomma: adding new data on Amblyomma maculatum and Amblyomma ovale species (Acari: Ixodidae) Fredy Arvey Rivera-Páez1,2 & Bruno Rodrigues Sampieri3 & Marcelo Bahia Labruna4 & Renata da Silva Matos1 & Thiago Fernandes Martins4 & Maria Izabel Camargo-Mathias1 Received: 9 May 2017 /Accepted: 10 August 2017 /Published online: 18 August 2017 # Springer-Verlag GmbH Germany 2017 Abstract Among tick species, members of the subfamily (mitochondrial) molecular markers were used to study the genet- Amblyomminae have received special attention, since they serve ic relationships of the species. The results show that the tick male as vectors for pathogens such as Rickettsia spp. and display reproductive system and its germ cells contain useful candidate cryptic species complexes that make their taxonomical classifi- characters for taxonomical analyses of Ixodida. cation challenging. Amblyomma ovale, Amblyomma maculatum, and other species of the genus Amblyomma have shown a long Keywords Cryptic species . Spermiotaxonomy . Ticks . history of taxonomic controversies. Spermiotaxonomy has Histology . Molecular markers proved to be a valuable tool in the solution of systematic con- flicts in Metazoa that can aid molecular and external morpholog- ical analyses in ticks and, overall, provide more robust analyses Introduction and results. With this in mind, this study included histological analyses of the reproductive system of the species A. ovale and Tick (Ixodidae) systematics has been discussed for decades, A. maculatum, as well as the description of morphohistological and periodically, the phylogeny of several groups is revisited characters of the male reproductive system of ticks of the genus through new analytical tools. -

Redalyc.Ticks Infesting Wild Small Rodents in Three Areas of the State Of
Ciência Rural ISSN: 0103-8478 [email protected] Universidade Federal de Santa Maria Brasil Fernandes Martins, Thiago; Gea Peres, Marina; Borges Costa, Francisco; Silva Bacchiega, Thais; Appolinario, Camila Michele; Azevedo de Paula Antunes, João Marcelo; Ferreira Vicente, Acácia; Megid, Jane; Bahia Labruna, Marcelo Ticks infesting wild small rodents in three areas of the state of São Paulo, Brazil Ciência Rural, vol. 46, núm. 5, mayo, 2016, pp. 871-875 Universidade Federal de Santa Maria Santa Maria, Brasil Available in: http://www.redalyc.org/articulo.oa?id=33144653018 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Ciência Rural, Santa Maria, v.46,Ticks n.5, infesting p.871-875, wild mai, small 2016 rodents in three areas of the state of http://dx.doi.org/10.1590/0103-8478cr20150671São Paulo, Brazil. 871 ISSN 1678-4596 PARASITOLOGY Ticks infesting wild small rodents in three areas of the state of São Paulo, Brazil Carrapatos infestando pequenos roedores silvestres em três municípios do estado de São Paulo, Brasil Thiago Fernandes MartinsI* Marina Gea PeresII Francisco Borges CostaI Thais Silva BacchiegaII Camila Michele AppolinarioII João Marcelo Azevedo de Paula AntunesII Acácia Ferreira VicenteII Jane MegidII Marcelo Bahia LabrunaI ABSTRACT carrapatos, os quais foram coletados e identificados ao nível de espécie em laboratório, através de análises morfológicas (para From May to September 2011, a total of 138 wild adultos, ninfas e larvas) e por biologia molecular para confirmar rodents of the Cricetidae family were collected in the cities of estas análises, através do sequenciamento de um fragmento Anhembi, Bofete and Torre de Pedra, in São Paulo State. -

Ehrlichiosis and Anaplasmosis Are Tick-Borne Diseases Caused by Obligate Anaplasmosis: Intracellular Bacteria in the Genera Ehrlichia and Anaplasma
Ehrlichiosis and Importance Ehrlichiosis and anaplasmosis are tick-borne diseases caused by obligate Anaplasmosis: intracellular bacteria in the genera Ehrlichia and Anaplasma. These organisms are widespread in nature; the reservoir hosts include numerous wild animals, as well as Zoonotic Species some domesticated species. For many years, Ehrlichia and Anaplasma species have been known to cause illness in pets and livestock. The consequences of exposure vary Canine Monocytic Ehrlichiosis, from asymptomatic infections to severe, potentially fatal illness. Some organisms Canine Hemorrhagic Fever, have also been recognized as human pathogens since the 1980s and 1990s. Tropical Canine Pancytopenia, Etiology Tracker Dog Disease, Ehrlichiosis and anaplasmosis are caused by members of the genera Ehrlichia Canine Tick Typhus, and Anaplasma, respectively. Both genera contain small, pleomorphic, Gram negative, Nairobi Bleeding Disorder, obligate intracellular organisms, and belong to the family Anaplasmataceae, order Canine Granulocytic Ehrlichiosis, Rickettsiales. They are classified as α-proteobacteria. A number of Ehrlichia and Canine Granulocytic Anaplasmosis, Anaplasma species affect animals. A limited number of these organisms have also Equine Granulocytic Ehrlichiosis, been identified in people. Equine Granulocytic Anaplasmosis, Recent changes in taxonomy can make the nomenclature of the Anaplasmataceae Tick-borne Fever, and their diseases somewhat confusing. At one time, ehrlichiosis was a group of Pasture Fever, diseases caused by organisms that mostly replicated in membrane-bound cytoplasmic Human Monocytic Ehrlichiosis, vacuoles of leukocytes, and belonged to the genus Ehrlichia, tribe Ehrlichieae and Human Granulocytic Anaplasmosis, family Rickettsiaceae. The names of the diseases were often based on the host Human Granulocytic Ehrlichiosis, species, together with type of leukocyte most often infected. -

An Insight Into the Ecobiology, Vector Significance and Control of Hyalomma Ticks (Acari: Ixodidae): a Review
Accepted Manuscript Title: AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW Authors: M.S. Sajid, A. Kausar, A. Iqbal, H. Abbas, Z. Iqbal, M.K. Jones PII: S0001-706X(18)30862-3 DOI: https://doi.org/10.1016/j.actatropica.2018.08.016 Reference: ACTROP 4752 To appear in: Acta Tropica Received date: 6-7-2018 Revised date: 10-8-2018 Accepted date: 12-8-2018 Please cite this article as: Sajid MS, Kausar A, Iqbal A, Abbas H, Iqbal Z, Jones MK, AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW, Acta Tropica (2018), https://doi.org/10.1016/j.actatropica.2018.08.016 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW M. S. SAJID 1 2 *, A. KAUSAR 3, A. IQBAL 4, H. ABBAS 5, Z. IQBAL 1, M. K. JONES 6 1. Department of Parasitology, Faculty of Veterinary Science, University of Agriculture, Faisalabad-38040, Pakistan. 2. One Health Laboratory, Center for Advanced Studies in Agriculture and Food Security (CAS-AFS) University of Agriculture, Faisalabad-38040, Pakistan. -

Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Spring 2009 Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences Erica Janelle Burkman Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Recommended Citation Burkman, Erica Janelle, "Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences" (2009). Electronic Theses and Dissertations. 704. https://digitalcommons.georgiasouthern.edu/etd/704 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. GENETIC STRUCTURE OF AMBLYOMMA CAJENNENSE (ACARI: IXODIDAE) POPULATIONS BASED ON MITOCHONDRIAL GENE SEQUENCES by ERICA JANELLE BURKMAN (Under the Direction of Dr. Lorenza Beati) ABSTRACT Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) is a common tick species that has a large geographic distribution from the southern regions of the United States (Texas), to the Caribbean Islands, Central, and South America. This tick is a vector of the agent of Brazilian spotted fever, an often fatal disease in South America. Throughout its geographic range, populations of A. cajennense have shown differences in ecological adaptation while feeding on a variety of hosts ranging from livestock, birds, and humans. In order to examine the taxonomic status and phylogeographic evolution of this species, we analyzed mitochondrial 12S rDNA, control region (d-loop), and cytochrome oxidase II gene sequences of A. -

Morphometrics of Amblyomma Mixtum in the State of Veracruz, Mexico
pathogens Article Morphometrics of Amblyomma mixtum in the State of Veracruz, Mexico Mariel Aguilar-Domínguez 1, Dora Romero-Salas 1,* , Sokani Sánchez-Montes 2, Ricardo Serna-Lagunes 3 , Greta Rosas-Saito 4, Anabel Cruz-Romero 1 and Adalberto A. Pérez de León 5,6 1 Laboratorio de Parasitología, rancho “Torreón del Molino”, Facultad de Medicina Veterinaria y Zootecnia, Universidad Veracruzana, Veracruz 91697, Mexico; [email protected] (M.A.-D.); [email protected] (A.C.-R.) 2 Facultad de Ciencias Biológicas y Agropecuarias Región Tuxpan, Universidad Veracruzana, Tuxpam 92870, Mexico; [email protected] 3 Laboratorio de Bioinformática y Bioestadística, Facultad de Ciencias Biológicas y Agropecuarias, Universidad Veracruzana, Córdoba 94945, Mexico; [email protected] 4 Red de Estudios Moleculares Avanzados, Instituto de Ecología, Xalapa 91073, Mexico; [email protected] 5 USDA-ARS Knipling-Bushland U.S. Veterinary Pest Genomics Center and Livestock Insects Research Laboratory, Kerrville, TX 78028, USA; [email protected] 6 USDA-ARS San Joaquin Valley Agricultural Sciences Center, Parlier, CA 93648, USA * Correspondence: [email protected]; Tel.: +52-(229)-9342075 Abstract: The tick Amblyomma mixtum is an ectoparasite of veterinary and public health importance because of its role as a vector of zoonotic pathogens. However, little is known about A. mixtum intraspecific variability and if morphological differentiation exists between populations across its geographic range. This study aimed to determine by electron microscopy the morphological vari- ability of A. mixtum populations in the state of Veracruz, which has a large livestock population Citation: Aguilar-Domínguez, M.; among states in Mexico. Forty male and 40 female A. mixtum collected from the 10 natural regions Romero-Salas, D.; Sánchez-Montes, S.; of Veracruz state were analyzed microscopically to accomplish main component analysis for each Serna-Lagunes, R.; Rosas-Saito, G.; sex. -

Ectoparasites Infesting Animals Living in Close Contact with Human Beings: a Real Trouble for One Health Perspective?
Arq. Bras. Med. Vet. Zootec., v.73, n.1, p.55-61, 2021 Ectoparasites infesting animals living in close contact with human beings: a real trouble for One Health perspective? [Ectoparasitos infestando animais que vivem em contato próximo com seres humanos: um problema real para a perspectiva Saúde Única?] J.C.P. Oliveira1, W.S.M. Oliveira2, R.S. Brito2, T.A.R.F. Lima3, 4 2 2* A. Giannelli , G.A. Carvalho , R.A.N. Ramos 1 Aluna de pós-graduação ˗ Universidade Federal Rural de Pernambuco ˗ Recife, PE 2 Universidade Federal do Agreste de Pernambuco, Garanhuns, PE 3 Universidade Federal de Pernambuco, Recife, PE 4 Vaxinano ˗ Faculté de Medecine ˗ Pole recherche ˗ Lille, França ABSTRACT The number of domestic animals living with human beings is rapidly increasing in parallel with an enhanced risk of transmission of their parasites and the pathogens they might carry. The aim of this study was to assess the occurrence of hematophagous arthropods infesting domestic animals from Northeastern Brazil and to remark the implications of their occurrence on the epidemiology and control of selected veterinary and human diseases. From January 2017 to April 2019, ectoparasites infesting domestic cats, dogs and horses were collected for their respective hosts and identified. Overall, ectoparasites were sampled from 86 domestic animals, living in different anthropic settings. A total of 401 specimens (344 ticks and 57 fleas) were collected from different hosts [i.e., 10 (2.49%), 96 (23.94%) and 295 (73.57%) from cats, dogs and horses, respectively]. Two flea (i.e., Ctenocephalides canis and Ctenocephalides felis) and 5 tick species (i.e., Amblyomma ovale, Amblyomma sculptum, Dermacentor nitens, Rhipicephalus microplus and Rhipicephalus sanguineus sensu lato) were identified. -

Acari: Ixodidae) Y Rol Potencial Como Vectores De Microorganismos Rickettsiales En Argentina
1 UNIVERSIDAD NACIONAL DEL LITORAL FACULTAD DE CIENCIAS VETERINARIAS ESPERANZA, SANTA FE, ARGENTINA Tesis presentada para acceder al grado académico de Doctor en Ciencias Veterinarias Aspectos ecológicos de Amblyomma tonelliae Nava, Beati y Labruna, 2014 y Amblyomma sculptum Berlese, 1888 (Acari: Ixodidae) y rol potencial como vectores de microorganismos rickettsiales en Argentina. MV. Evelina Luisa Tarragona Director: Dr. Santiago Nava Co-Director: Dr. Pablo M Beldoménico Laboratorio de Parasitología e Inmunología Estación Experimental Rafaela Instituto Nacional de Tecnología Agropecuaria (INTA) 2017 2 “La ciencia no tiene patria, pero el hombre de ciencia sí la tiene” (Bernardo Alberto Houssay) 3 Agradecimientos Los resultados obtenidos de este trabajo de tesis doctoral sin duda no son logro de una persona, sino más bien, de un grupo de personas que han sumado dedicación para lograrlo y de instituciones dispuestas a solventar los gastos que la realización del trabajo demandó. Es por ello que quisiera expresar mi agradecimiento a cada uno de los que en mayor o menor medida contribuyeron. En primer lugar, quiero expresar mi mayor agradecimiento al Dr. Santiago Nava por su apoyo y acompañamiento como director de esta tesis doctoral y sobre todo por el extraordinario talento y dedicación por el trabajo que ha sabido transmitir. En segundo lugar, al Dr. Alberto Alejandro Guglielmone por haberme aceptado como becaria de posgrado bajo su dirección. Su visión crítica y su humildad científica han enriquecido mi formación. Un profundo agradecimiento al Dr. Pablo Martín Beldoménico por haber aceptado ser el co-director de esta tesis doctoral y por haberme introducido, siendo estudiante de grado, en el fascinante mundo de la ciencia. -

MORFOLOGIA E DESENVOLVIMENTO DO SISTEMA REPRODUTOR MASCULINO DE CARRAPATOS DO GÊNERO Amblyomma (ACARI, IXODIDAE): UMA ANÁLISE COMPARATIVA
UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” INSTITUTO DE BIOCIÊNCIAS – RIO unesp CLARO PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS (BIOLOGIA CELULAR E MOLECULAR) MORFOLOGIA E DESENVOLVIMENTO DO SISTEMA REPRODUTOR MASCULINO DE CARRAPATOS DO GÊNERO Amblyomma (ACARI, IXODIDAE): UMA ANÁLISE COMPARATIVA BRUNO RODRIGUES SAMPIERI Tese apresentada ao Instituto de Biociências do Campus de Rio Claro, Universidade Estadual Paulista, como parte dos requisitos para obtenção do título de Doutor em Ciências Biológicas (Biologia Celular e Molecular). Maio - 2016 UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” INSTITUTO DE BIOCIÊNCIAS – RIO unesp CLARO PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS (BIOLOGIA CELULAR E MOLECULAR) MORFOLOGIA E DESENVOLVIMENTO DO SISTEMA REPRODUTOR MASCULINO DE CARRAPATOS DO GÊNERO Amblyomma (ACARI, IXODIDAE): UMA ANÁLISE COMPARATIVA BRUNO RODRIGUES SAMPIERI ORIENTADORA: PROFA. DRA. MARIA IZABEL CAMARGO-MATHIAS CO-ORIENTADOR: PROF. DR. ODAIR CORREA BUENO Tese apresentada ao Instituto de Biociências do Campus de Rio Claro, Universidade Estadual Paulista, como parte dos requisitos para obtenção do título de Doutor em Ciências Biológicas (Biologia Celular e Molecular). Maio - 2016 591.4 Sampieri, Bruno Rodrigues S192m Morfologia e desenvolvimento do sistema reprodutor masculino de carrapatos do gênero Amblyomma (Acari, Ixodidae) : uma análise comparativa / Bruno Rodrigues Sampieri. - Rio Claro, 2016 113 f. : il., figs., tabs. Tese (doutorado) - Universidade Estadual Paulista, Instituto de Biociências de Rio Claro Orientadora: Maria Izabel Souza Camargo Coorientador: Odair Correa Bueno 1. Anatomia comparada. 2. Espermiotaxonomia. 3. Espermiocladística. 4. Filogenia. 5. Controle. I. Título. Ficha Catalográfica elaborada pela STATI - Biblioteca da UNESP Campus de Rio Claro/SP Dedicatória Dedico esta tese aos meus pais, à minha esposa, à minha filha e ao meu irmão, pelo apoio, companheirismo e paciência; por compartilharem comigo angústias e conquistas. -
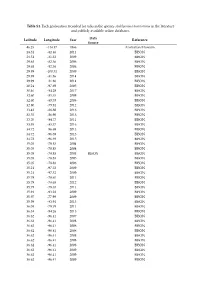
Table S1. Each Geolocation Recorded for Ticks in the Species Amblyomma Americanum in the Literature and Publicly Available Online Databases
Table S1. Each geolocation recorded for ticks in the species Amblyomma americanum in the literature and publicly available online databases. Data Latitude Longitude Year Reference Source 46.25 -114.17 1966 Australian Museum 28.31 -82.46 2011 BISON 28.51 -81.32 2009 BISON 29.68 -82.36 2006 BISON 29.68 -82.36 2006 BISON 29.99 -100.31 2009 BISON 29.99 -81.86 2014 BISON 29.99 -81.86 2014 BISON 30.24 -97.69 2005 BISON 30.46 -84.28 2017 BISON 32.60 -85.35 2008 BISON 32.60 -85.35 2008 BISON 32.80 -79.94 2012 BISON 33.42 -88.88 2016 BISON 33.55 -86.90 2013 BISON 33.70 -84.77 2011 BISON 33.95 -83.37 2016 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2015 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON BISON 35.05 -78.83 2005 BISON 35.05 -78.83 2006 BISON 35.21 -97.32 2009 BISON 35.21 -97.32 2009 BISON 35.79 -78.65 2011 BISON 35.79 -78.65 2012 BISON 35.79 -78.65 2011 BISON 35.91 -93.22 2009 BISON 35.97 -77.99 2009 BISON 35.99 -83.94 2015 BISON 36.08 -79.79 2011 BISON 36.34 -94.26 2015 BISON 36.62 -96.41 2007 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2011 BISON 36.62 -96.41 2012 BISON 36.62 -96.41 2012 BISON 37.36 -77.05 2014 BISON 37.62 -84.87 2016 BISON 37.83 -78.28 2011 BISON 37.97 -85.70 2016 BISON 37.97 -85.70 2016 BISON 38.22 -75.31 2014 BISON 38.43 -88.43 -

2.2. Piroplasmosis Equina
UNIVERSIDAD NACIONAL DE LOJA FACULTAD AGROPECUARIA Y DE RECURSOS NATURALES RENOVABLES CARRERA DE MEDICINA VETERINARIA Y ZOOTECNIA DETERMINACI ÓN DE LA FRECUENCIA DE PIROPLASMOSIS EQUINA Y DE LOS VECTORES IMPLICADOS EN LA TRANSMISIÓN, EN EL CANTÓN CATAMAYO, PROVINCIA DE LOJA Trabajo de tesis previo a la obtención del título de MÉDICA VETERINARIA ZOOTECNISTA Autor: Stefany Yadira Lapo Rojas Director: MVZ. Jhuliana Katherine Luna Herrera, Mg. Sc. LOJA - ECUADOR 2019 ii iii iv v AGRADECIMIENTOS Agradezco a Dios por brindarme salud, vida, fortaleza y perseverancia, gracias a ello culmine satisfactoriamente mis estudios universitarios y pude realizar la presente investigación exitosamente. Mi sincero agradecimiento a mi familia por el incondicional apoyo moral y económico que supieron darme durante mi vida estudiantil, sin ellos este logro no fuese posible, gracias por ayudarme a cristalizar mis sueños, los quiero mucho. Le agradezco a mi directora de tesis, quien supo guiarme y ayudarme durante la realización de esta investigación, gracias por su paciencia Doc. Jhuly. Stefany Yadira Lapo Rojas vi DEDICATORIA A mi bella madre Luz María que desde el cielo siempre me guía y me cuida Stefany Yadira Lapo Rojas vii ÍNDICE GENERAL ÍNDICE DE TABLAS x ÍNDICE DE FIGURAS xii RESUMEN xiv ABSTRACT xv 1. INTRODUCCIÓN .................................................................................... 1 2. REVISIÓN DE LITERATURA .............................................................. 3 2.1. SANIDAD EQUINA .............................................................................