High-Arctic Butterflies Become Smaller with Rising Temperatures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Likely to Have Habitat Within Iras That ALLOW Road
Item 3a - Sensitive Species National Master List By Region and Species Group Not likely to have habitat within IRAs Not likely to have Federal Likely to have habitat that DO NOT ALLOW habitat within IRAs Candidate within IRAs that DO Likely to have habitat road (re)construction that ALLOW road Forest Service Species Under NOT ALLOW road within IRAs that ALLOW but could be (re)construction but Species Scientific Name Common Name Species Group Region ESA (re)construction? road (re)construction? affected? could be affected? Bufo boreas boreas Boreal Western Toad Amphibian 1 No Yes Yes No No Plethodon vandykei idahoensis Coeur D'Alene Salamander Amphibian 1 No Yes Yes No No Rana pipiens Northern Leopard Frog Amphibian 1 No Yes Yes No No Accipiter gentilis Northern Goshawk Bird 1 No Yes Yes No No Ammodramus bairdii Baird's Sparrow Bird 1 No No Yes No No Anthus spragueii Sprague's Pipit Bird 1 No No Yes No No Centrocercus urophasianus Sage Grouse Bird 1 No Yes Yes No No Cygnus buccinator Trumpeter Swan Bird 1 No Yes Yes No No Falco peregrinus anatum American Peregrine Falcon Bird 1 No Yes Yes No No Gavia immer Common Loon Bird 1 No Yes Yes No No Histrionicus histrionicus Harlequin Duck Bird 1 No Yes Yes No No Lanius ludovicianus Loggerhead Shrike Bird 1 No Yes Yes No No Oreortyx pictus Mountain Quail Bird 1 No Yes Yes No No Otus flammeolus Flammulated Owl Bird 1 No Yes Yes No No Picoides albolarvatus White-Headed Woodpecker Bird 1 No Yes Yes No No Picoides arcticus Black-Backed Woodpecker Bird 1 No Yes Yes No No Speotyto cunicularia Burrowing -

Effects of Climate Change on Arctic Arthropod Assemblages and Distribution Phd Thesis
Effects of climate change on Arctic arthropod assemblages and distribution PhD thesis Rikke Reisner Hansen Academic advisors: Main supervisor Toke Thomas Høye and co-supervisor Signe Normand Submitted 29/08/2016 Data sheet Title: Effects of climate change on Arctic arthropod assemblages and distribution Author University: Aarhus University Publisher: Aarhus University – Denmark URL: www.au.dk Supervisors: Assessment committee: Arctic arthropods, climate change, community composition, distribution, diversity, life history traits, monitoring, species richness, spatial variation, temporal variation Date of publication: August 2016 Please cite as: Hansen, R. R. (2016) Effects of climate change on Arctic arthropod assemblages and distribution. PhD thesis, Aarhus University, Denmark, 144 pp. Keywords: Number of pages: 144 PREFACE………………………………………………………………………………………..5 LIST OF PAPERS……………………………………………………………………………….6 ACKNOWLEDGEMENTS……………………………………………………………………...7 SUMMARY……………………………………………………………………………………...8 RESUMÉ (Danish summary)…………………………………………………………………....9 SYNOPSIS……………………………………………………………………………………....10 Introduction……………………………………………………………………………………...10 Study sites and approaches……………………………………………………………………...11 Arctic arthropod community composition…………………………………………………….....13 Potential climate change effects on arthropod composition…………………………………….15 Arctic arthropod responses to climate change…………………………………………………..16 Future recommendations and perspectives……………………………………………………...20 References………………………………………………………………………………………..21 PAPER I: High spatial -

Åâðàçèàòñêèé Ýíòîìîëîãè÷Åñêèé Æóðíàë
Ñèáèðñêîå îòäåëåíèå Ðîññèéñêîé àêàäåìèè íàóê Èíñòèòóò ñèñòåìàòèêè è ýêîëîãèè æèâîòíûõ ÑÎ ÐÀÍ Òîâàðèùåñòâî íàó÷íûõ èçäàíèé ÊÌÊ Åâðàçèàòñêèé Ýíòîìîëîãè÷åñêèé Æóðíàë Euroasian Entomological Journal Òîì 18. Âûï. 6 Vol. 18. No. 6 Äåêàáðü 2019 December 2019 Íîâîñèáèðñê–Ìîñêâà 2019 Отдел подписки: К.Г. Михайлов Distribution manager: K.G. Mikhailov Fax (7-495) 203-2717 E-mail: [email protected] Адресa для переписки: Сергей Эдуардович Чернышёв ИСиЭЖ СО РАН, ул. Фрунзе 11, Новосибирск 630091 Россия. E-mail: [email protected] Кирилл Глебович Михайлов Зоологический музей МГУ, ул. Большая Никитская 6, Москва 125009 Россия. E-mail: [email protected] Addresses for correspondence: Dr. S.E. Tshernyshev, Institute of Systematics and Ecology of Animals, Russian Academy of Sciences, Siberian Branch, Frunze str. 11, Novosibirsk 630091 Russia Dr. K.G. Mikhailov, Zoological Museum of the Moscow State University, Bolshaya Nikitskaya str. 6, Moscow 125009 Russia Отпечатано в сентябре 2019 г. Printed in September 2019 Рецензируемый научный журнал На обложке и титуле — Lepyrus volgensis (Faust, 1882). Фото С.В. Решетникова. Информация о журнале и правила для авторов доступны в интернете по адресам: http://www.eco.nsc.ru/entomolog.html, www.eej.su Information on the Journal is available in web sites: http://www.eco.nsc.ru/entomolog.html, www.eej.su Техническое редактирование и вёрстка — О.Г. Березина, корректура — Е.В. Зинченко © «Евразиатский энтомологический журнал», 2019 — составление, редактирование compiling, editing © В.В. Глупов (V.V. Glupov), 2019 — макет обложки cover design Евразиатский энтомол. журнал 18(6): 379–381 © EUROASIAN ENTOMOLOGICAL doi: 10.15298/euroasentj.18.6.1 JOURNAL, 2019 First records of the entomopathogenic fungus Ophiocordyceps variabilis (Petch) G.H. -

Behavioural Thermoregulation by High Arctic Butterflies*
Behavioural Thermoregulation by High Arctic Butterflies* P. G. KEVAN AND J. D. SHORTHOUSE2s ABSTRACT. Behavioural thermoregulation is an important adaptation of the five high arctic butterflies found at Lake Hazen (81 “49‘N., 71 18’W.), Ellesmere Island, NorthwestTerritories. Direct insolation is used byarctic butterflies to increase their body temperatures. They select basking substrates andprecisely orientate their wings with respect to the sun. Some experiments illustrate the importance of this. Wing morphology, venation, colour, hairiness, and physiology are briefly discussed. RI~SUMÉ.Comportement thermo-régulatoire des papillons du haut Arctique. Chez cinq especes de papillons trouvés au lac Hazen (81”49’ N, 71” 18’ W), île d’Elles- mere, Territoires du Nord-Ouest, le comportement thermo-régulatoire est une im- portante adaptation. Ces papillons arctiques se servent de l’insolation directe pour augmenter la température de leur corps: ils choisissent des sous-strates réchauffantes et orientent leurs ailes de façon précise par rapport au soleil. Quelques expériences ont confirmé l’importance de ce fait. On discute brikvement de la morphologie alaire, de la couleur, de la pilosité et de la physiologie de ces insectes. INTRODUCTION Basking in direct sunlight has long been known to have thermoregulatory sig- nificance for poikilotherms (Gunn 1942), particularly reptiles (Bogert 1959) and desert locusts (Fraenkel 1P30;.Stower and Griffiths 1966). Clench (1966) says that this was not known before iwlepidoptera but Couper (1874) wrote that the common sulphur butterfly, Colias philodice (Godart) when resting on a flower leans sideways “as if to receive the warmth of the sun”. Later workers, noting the consistent settling postures and positions of many butterflies, did not attribute them to thermoregulation, but rather to display (Parker 1903) or concealment by shadow minimization (Longstaff 1905a, b, 1906, 1912; Tonge 1909). -

Yukon Butterflies a Guide to Yukon Butterflies
Wildlife Viewing Yukon butterflies A guide to Yukon butterflies Where to find them Currently, about 91 species of butterflies, representing five families, are known from Yukon, but scientists expect to discover more. Finding butterflies in Yukon is easy. Just look in any natural, open area on a warm, sunny day. Two excellent butterfly viewing spots are Keno Hill and the Blackstone Uplands. Pick up Yukon’s Wildlife Viewing Guide to find these and other wildlife viewing hotspots. Visitors follow an old mining road Viewing tips to explore the alpine on top of Keno Hill. This booklet will help you view and identify some of the more common butterflies, and a few distinctive but less common species. Additional species are mentioned but not illustrated. In some cases, © Government of Yukon 2019 you will need a detailed book, such as , ISBN 978-1-55362-862-2 The Butterflies of Canada to identify the exact species that you have seen. All photos by Crispin Guppy except as follows: In the Alpine (p.ii) Some Yukon butterflies, by Ryan Agar; Cerisy’s Sphynx moth (p.2) by Sara Nielsen; Anicia such as the large swallowtails, Checkerspot (p.2) by Bruce Bennett; swallowtails (p.3) by Bruce are bright to advertise their Bennett; Freija Fritillary (p.12) by Sonja Stange; Gallium Sphinx presence to mates. Others are caterpillar (p.19) by William Kleeden (www.yukonexplorer.com); coloured in dull earth tones Butterfly hike at Keno (p.21) by Peter Long; Alpine Interpretive that allow them to hide from bird Centre (p.22) by Bruce Bennett. -

PIERIDAE) AIDED by ULTRAVIOLET REFLECTANCE PHOTOGRAPHY with DESIGNATION of a NEW Subspeciesl
VOLUME 27, NUMBER 1 57 LINDSEY, A. W . 1921. Hesperioidea of America, north of Mexico. Univ. Iowa Studies Nat. Hist. 9 : 1-114. 1928. Hesperia eos Edwards. Entomol. News 39: 91-93. ---, E . L. BELL & R. C. WILLIAMS, JR. 1931. The Hesperioidea of North America. Denison Univ. Bull. 31(2); (subtitle). J. Sci. Labs. 26(1): 1-142. 33 pIs. McDuNNOUGH, J. 1938. Check List of the Lepidoptera of Canada and the United States of America. Part 1, Macrolepidoptera. Memoirs S. Calif. Acad. Sci. 1. 275 p. SEITZ, A., ed. 1907-1924. The Macrolepidoptera of the World. Vol. V., The American Rhopalocera. Stuttgart. 1139 p., 203 pIs. Various authors. SKINNER, H. & R. C. WILLIAMS, JR. 1923. On the Male Genitalia of the Hesperiidae of North America. Paper III. Trans. Amer. Entomol. Soc. 49: 129-153. A REVISION OF THE COLlAS ALEXANDRA COMPLEX (PIERIDAE) AIDED BY ULTRAVIOLET REFLECTANCE PHOTOGRAPHY WITH DESIGNATION OF A NEW SUBSPECIESl CLIFFORD D. FERRIS2 College of Engineering, University of Wyoming, Laramie, Wyoming 82070 This paper presents a study of the distribution and taxonomy of the Colias alexandra complex. The role of ultraviolet photography as an aid to taxonomic studies is discussed and is employed in assigning C. alexandra populations to various color groups. Visible light characters (pigmentation and facies) are combined with uv reflectance patterns to arrive at the taxonomic conclusions presented. One concludes from this study that some populations of alexandra can be assigned to specific sub species, while others are best listed as clinal or intergrade forms. Based upon uv photography, C. harfordii and C. -

Butterflies and Moths of Yukon-Koyukuk, Alaska, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -
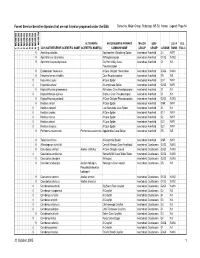
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

Insect Pollinators of Gates of the Arctic NPP a Preliminary Survey of Bees (Hymenoptera: Anthophila) and Flower Flies (Diptera: Syrphidae)
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Insect Pollinators of Gates of the Arctic NPP A Preliminary Survey of Bees (Hymenoptera: Anthophila) and Flower Flies (Diptera: Syrphidae) Natural Resource Report NPS/GAAR/NRR—2017/1541 ON THE COVER Left to right, TOP ROW: Bumble bee on Hedysarum, Al Smith collecting bees at Itkillik River; MIDDLE ROW: Al Smith and Just Jensen collecting pollinators on Krugrak River, Andrena barbilabris on Rosa; BOTTOM ROW: syrphid fly on Potentilla, bee bowl near Lake Isiak All photos by Jessica Rykken Insect Pollinators of Gates of the Arctic NPP A Preliminary Survey of Bees (Hymenoptera: Anthophila) and Flower Flies (Diptera: Syrphidae) Natural Resource Report NPS/GAAR/NRR—2017/1541 Jessica J. Rykken Museum of Comparative Zoology Harvard University 26 Oxford Street, Cambridge, MA 02138 October 2017 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. The series supports the advancement of science, informed decision-making, and the achievement of the National Park Service mission. The series also provides a forum for presenting more lengthy results that may not be accepted by publications with page limitations. -

Fritillary, White Mountain
Appendix A: Insects White Mountain Fritillary Boloria titania montinus Federal Listing N/A State Listing E Global Rank State Rank S1 Regional Status Photo by © K.P. McFarland Justification (Reason for Concern in NH) White Mountain fritillary is limited to the 2,800 ac alpine zone of the White Mountain National Forest (WMNF). The natural communities used most frequently by White Mountain fritillary ranked S1 in New Hampshire. Climate change will likely alter alpine habitat structure, composition, phenology, and distribution, all of which directly impact White Mountain fritillary populations (Kimball and Weihrauch 2000, McFarland 2003, Lesica and McCune 2004). Habitat isolation further increases the species’ vulnerability (Halloy and Mark 2003, McFarland 2003). Interdependent responses to climate change could disrupt ecological interactions throughout the alpine community, reducing the ability of sensitive species to endure other environmental stresses, such as acid deposition and increased UV‐B radiation (McCarty 2001). Distribution White Mountain fritillary is a subspecies endemic to the 2,800 ac alpine zone of the Presidential Range of the WMNF (McFarland 2003). Habitat suitability depends on the abundance of host plants, particularly Alpine goldenrod, as well as ground temperature, moisture, and winter snow cover (Anthony 1970, McFarland 2003). White Mountain fritillary populations tend to be locally abundant, the northernmost occurrence is from Mt. Madison and the southernmost is Mt. Pierce at an elevation range of 1,220 to 1,860 m, with the highest densities at Cragway Spring and Wamsutta Trail (McFarland 2003). The only historical record occurring outside the Presidential Range alpine zone was a specimen collected by D. J. -

A Primary Insight Into the Molecular Phylogeny of Colias FABRICIUS, 1807 (Pieridae, Coliadinae) Complex of South America
A primary insight into the molecular phylogeny of Colias FABRICIUS, 1807 (Pieridae, Coliadinae) complex of South America Alexander V. Kir’yanov Centro de Investigaciones en Optica Loma del bosque 115, Col. Lomas del Campestre, Leon 37150, GTO., Mexico [email protected] Abstract The data on molecular phylogeny of the Colias FABRICIUS complex of South America (SA), obtained via barcoding a mitochondrial part of genome, are reported. Barcoding was trialed employing the Barcoding of Life Database platform and comprised 93 specimens of SA Colias species and 2 outgroup specimens (C. philodice guatemalena RÖBER from North America). It is established that Colias species from SA form a single monophyletic clade, characterized by notable interspecific mutational divergences (3.5–4.5%) and moderate intraspecific ones (0.3–0.9%). It also reveals the occurrence of three well-established, or notably diverged genetically, species (C. dimera DOUBLEDAY & HEWITSON, C. vauthierii GUERIN-MENEVILLE, and C. alticola GODMANN & SALVIN stat. nov., the latter re-raised to specific level), plus a complex of three ‘emerging’ species (C. lesbia FABRICIUS, C. euxanthe FELDER & FELDER, and C. flaveola BLANCHARD), weakly differentiated in mitochondrial part of genome, with intersecting haplotypes. Furthermore, it disregards the specific status of taxa weberbaueri STRAND, mossi ROTHSCHILD, nigerrima FASSL, erika LAMAS, blameyi JÖRGENSEN, and mendozina BREYER, as certain evidences are being brought to consider them as subspecies of a clinal type of ‘super-species’ C. flaveola s. lat. The clade formed by all SA Colias is found to be sister to the clade that comprises all Colias species occurring outside the region (i.e. in Eurasia, North America, and Africa). -

Blue Jay, Vol.52, Issue 2
INSECTS BUTTERFLIES OF THE PEACE RIVER REGION OF ALBERTA AND BRITISH COLUMBIA NORBERT G. KONDLA, British Columbia Forest Service, Box 672, McBride, British Columbia, VOJ 6E0, EDWARD M. PIKE, Department of Biological Sciences, University of Calgary, Calgary, Alberta, T2N 1N4, and FELIX A. H. SPERLING, Department of Biology, University of Ottawa, Ottawa, Ontario. K1N 6N5 Introduction Although the Peace to 1986 and was the first to find River valley and surrounding area many of the interesting butterfly taxa were explored early in Alberta’s his¬ resident in this area. Sperling ex¬ tory and have been settled since the plored the region while conducting early 1900s, little has been published research on the genus Papilio from regarding its insect fauna.20 Works 1980 to 1986. Kondla conducted ex¬ describing various aspects of the tensive surveys over eight years natural history of the Peace River from 1979 to 1992. We also include valley include Spalding with a gen¬ records provided by K. Avery, C. eral synthesis, Moss on plants, and Schmidt, C. Guppy, G.J. Hilchie, J. Soper on mammals and Pelham, A.W. Rupp and J. Shepard. birds.25'26’36'37'38 The primary purpose of this paper is to report on the results of this in¬ With respect to butterflies, Llewel- creased activity since 1979. lyn-Jones lists 12 species from the Peace River district of British Colum¬ The study area we deal with herein bia, and Bowman mentions six spe¬ consists of the Peace River valley cies from the Peace River district of from Bullhead Mountain/ Dunlevy Alberta.1,21 Case