Chapter 9 • Marine Systems 455
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Matthew Henson (August 8, 1866 – March 9, 1955) “First African-American Artic Explorer”
The Clerk’s Black History Series Debra DeBerry Clerk of Superior Court DeKalb County Matthew Henson (August 8, 1866 – March 9, 1955) “First African-American Artic Explorer” Matthew Henson was born August 8, 1866, in Nanjemoy, Maryland, to freeborn black sharecropper parents. In 1867, his parents and three sisters moved to Georgetown to escape racial violence where his mother died when Matthew was seven years old. When Matthew’s father died, he went to live with his uncle in Washington, D.C. When Matthew was ten years old, he attended a ceremony honoring Abraham Lincoln where he heard social reformer and abolitionist, Frederick Douglas speak. Shortly thereafter, he left home, determined to find his own way. After working briefly in a restaurant, he walked all the way to Baltimore, Maryland. At the age of 12, Matthew went to sea as a cabin boy on the merchant ship Katie Hines, traveling to Asia, Africa and Europe under the watchful eye of the ship’s skipper, Captain Childs. After Captain Childs died, Matthew moved back to Washington, D.C. When Matthew was 21 years old, he met Commander Robert E. Peary, an explorer and officer in the U.S. Navy Corps of Civil Engineers. Impressed with Matthew’s seafaring experience, Commander Peary recruited him for an upcoming voyage to Nicaragua. After returning from Nicaragua, Matthew found work in Philadelphia, and in April 1891 he met and married Eva Flint. But shortly thereafter, the two explorers were off again for an expedition to Green- land and the marriage to Eva ended. Matthew and the Commander would cover thousands of miles across the sea and the world, exploring and making multiple attempts to reach the North Pole. -

Internal Frost Damage in Concrete - Experimental Studies of Destruction Mechanisms
Internal frost damage in concrete - experimental studies of destruction mechanisms Fridh, Katja 2005 Link to publication Citation for published version (APA): Fridh, K. (2005). Internal frost damage in concrete - experimental studies of destruction mechanisms. Division of Building Materials, LTH, Lund University. Total number of authors: 1 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 Download date: 09. Oct. 2021 LUND INSTITUTE OF TECHNOLOGY LUND UNIVERSITY Division of Building Materials INTERNAL FROST DAMAGE IN CONCRETE Experimental studies of destruction mechanisms Katja Fridh Report TVBM-1023 Doctoral thesis Lund 2005 ISRN LUTVDG/TVBM--05/1023--SE(1-276) ISSN 0348-7911 TVBM ISBN 91-628-6558-7 Lund Institute of Technology Telephone: 46-46-2227415 Division of Building Materials Telefax: 46-46-2224427 Box 118 www.byggnadsmaterial.lth.se SE-221 00 Lund, Sweden Preface The work presented here was carried out at the Division of Building Materials at the Lund Institute of Technology. -

Special Supplement to the Bulletin of the American Meteorological Society Vol
J. Blunden, D. S. Arndt, and M. O. Baringer, Eds. Associate Eds. K. M. Willett, A. J. Dolman, B. D. Hall, P. W. Thorne, J. M. Levy, H. J. Diamond, J. Richter-Menge, M. Jeffries, R. L. Fogt, L. A. Vincent, and J. M. Renwick Special Supplement to the Bulletin of the American Meteorological Society Vol. 92, No. 6, June 2011 www.whoi.edu/beaufort) show that the pack ice in the e. Land central Canada Basin is changing from a multiyear to 1) veGetation—D. A. Walker, U. S. Bhatt, T. V. Callaghan, J. a seasonal ice cover. C. Comiso, H. E. Epstein, B. C. Forbes, M. Gill, W. A. Gould, G. H. R. Henry, G. J. Jia, S. V. Kokelj, T. C. Lantz, S. F Oberbauer, 3) Sea ice thickness J. E. Pinzon, M. K. Raynolds, G. R. Shaver, C. J. Tucker, C. E. Combined estimates of ice thickness from sub- Tweedie, and P. J. Webber marine and satellite-based instruments provide the Circumpolar changes to tundra vegetation are longest record of sea ice thickness observation, begin- monitored from space using the Normalized Differ- ning in 1980 (Kwok et al. 2009; Ro throck et al. 2008). ence Vegetation Index (NDVI), an index of vegetation These data indicate that over a region covering ~38% greenness. In tundra regions, the annual maximum of the Arctic Ocean there is a long-term trend of sea NDVI (MaxNDVI) is usually achieved in early Au- ice thinning over the last three decades. gust and is correlated with above-ground biomass, Haas et al. -

The Eu and the Arctic
MAGAZINE Dealing the Seal 8 No. 1 Piloting Arctic Passages 14 2016 THE CIRCLE The EU & Indigenous Peoples 20 THE EU AND THE ARCTIC PUBLISHED BY THE WWF GLOBAL ARCTIC PROGRAMME TheCircle0116.indd 1 25.02.2016 10.53 THE CIRCLE 1.2016 THE EU AND THE ARCTIC Contents EDITORIAL Leaving a legacy 3 IN BRIEF 4 ALYSON BAILES What does the EU want, what can it offer? 6 DIANA WALLIS Dealing the seal 8 ROBIN TEVERSON ‘High time’ EU gets observer status: UK 10 ADAM STEPIEN A call for a two-tier EU policy 12 MARIA DELIGIANNI Piloting the Arctic Passages 14 TIMO KOIVUROVA Finland: wearing two hats 16 Greenland – walking the middle path 18 FERNANDO GARCES DE LOS FAYOS The European Parliament & EU Arctic policy 19 CHRISTINA HENRIKSEN The EU and Arctic Indigenous peoples 20 NICOLE BIEBOW A driving force: The EU & polar research 22 THE PICTURE 24 The Circle is published quar- Publisher: Editor in Chief: Clive Tesar, COVER: terly by the WWF Global Arctic WWF Global Arctic Programme [email protected] (Top:) Local on sea ice in Uumman- Programme. Reproduction and 8th floor, 275 Slater St., Ottawa, naq, Greenland. quotation with appropriate credit ON, Canada K1P 5H9. Managing Editor: Becky Rynor, Photo: Lawrence Hislop, www.grida.no are encouraged. Articles by non- Tel: +1 613-232-8706 [email protected] (Bottom:) European Parliament, affiliated sources do not neces- Fax: +1 613-232-4181 Strasbourg, France. sarily reflect the views or policies Design and production: Photo: Diliff, Wikimedia Commonss of WWF. Send change of address Internet: www.panda.org/arctic Film & Form/Ketill Berger, and subscription queries to the [email protected] ABOVE: Sarek glacier, Sarek National address on the right. -

The Maritime Dimension of Csdp
DIRECTORATE-GENERAL FOR EXTERNAL POLICIES OF THE UNION DIRECTORATE B POLICY DEPARTMENT STUDY THE MARITIME DIMENSION OF CSDP: GEOSTRATEGIC MARITIME CHALLENGES AND THEIR IMPLICATIONS FOR THE EUROPEAN UNION Abstract The global maritime security environment is in the midst of an important transformation, driven by a simultaneous intensification of global maritime flows, the growing interconnectedness of maritime regions, the diffusion of maritime power to emerging powers, and the rise of a number of maritime non-state actors. These changes are having a profound impact on the maritime security environment of the EU and its member states and require an upgrading of the maritime dimension of the EU’s Common Security and Defence Policy (CSDP). This study analysis the impact that the changing maritime security context is having on the EU’s maritime neighbourhood and along the EU’s sea lines of communications (SLOCs) and takes stock of the EU’s existing policies and instruments in the maritime security domain. Based on this analysis, the study suggests that the EU requires a comprehensive maritime security strategy that creates synergies between the EU’s Integrated Maritime Policy and the maritime dimension of CSDP and that focuses more comprehensively on the security and management of global maritime flows and sea-based activities in the global maritime commons. EP/EXPO/B/SEDE/FWC/2009-01/Lot6/21 January 2013 PE 433.839 EN Policy Department DG External Policies This study was requested by the European Parliament's Subcommittee on Security and -

Arctic Policy &
Arctic Policy & Law References to Selected Documents Edited by Wolfgang E. Burhenne Prepared by Jennifer Kelleher and Aaron Laur Published by the International Council of Environmental Law – toward sustainable development – (ICEL) for the Arctic Task Force of the IUCN Commission on Environmental Law (IUCN-CEL) Arctic Policy & Law References to Selected Documents Edited by Wolfgang E. Burhenne Prepared by Jennifer Kelleher and Aaron Laur Published by The International Council of Environmental Law – toward sustainable development – (ICEL) for the Arctic Task Force of the IUCN Commission on Environmental Law The designation of geographical entities in this book, and the presentation of material, do not imply the expression of any opinion whatsoever on the part of ICEL or the Arctic Task Force of the IUCN Commission on Environmental Law concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers and boundaries. The views expressed in this publication do not necessarily reflect those of ICEL or the Arctic Task Force. The preparation of Arctic Policy & Law: References to Selected Documents was a project of ICEL with the support of the Elizabeth Haub Foundations (Germany, USA, Canada). Published by: International Council of Environmental Law (ICEL), Bonn, Germany Copyright: © 2011 International Council of Environmental Law (ICEL) Reproduction of this publication for educational or other non- commercial purposes is authorized without prior permission from the copyright holder provided the source is fully acknowledged. Reproduction for resale or other commercial purposes is prohibited without the prior written permission of the copyright holder. Citation: International Council of Environmental Law (ICEL) (2011). -
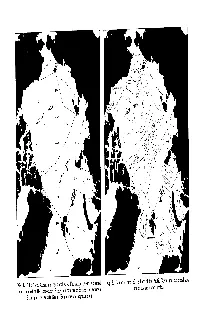
Geographic Names
Commentary THE FLOOR OF THE ARCTICOCEAN: GEOGRAPHIC NAMES M. A. Beall, F. Edvalson2, K. Hunkins3, A. Molloyl, and N. Ostenso4 HE NAMING OF OCEAN FLOOR features in the Arctic seems to have been done Twithout thought to standardization in geographic nomenclature. W. K. Lyon, Director of the Arctic Sciences and Technology Division of the US.Navy Electronics Laboratory, called a meeting of the authors of the present paper to arbitrate the naming of these features in the hope that it would help to stem the proliferation of new namesand to standardize the names of the major features. The decisions of the meeting which was held in San Diego on 10 and 11 January 1966 were based on the following criteria: 1. Consistency with the Undersea terms and definitions proposed by the Advisory Committee on Undersea Features to the U.S. Board on Geo- graphicNames (Washington, D.C.: 1964) andLimits of Oceans and Seas, InternationalHydrographic Bureau Special PublicationNo. 23 (Monte Carlo: 1953) 2. Common usage 3. Priority of discovery or naming 4. Association withestablished geographic features 5. Minimizing ambiguity Fifty-four major features were discussed. Table 1 lists the names considered, the final suggested name, the approximate location, and the status of the name with the U.S. Board on Geographic Names and the International Hydrographic Bureau. The final suggested names represent the majority decision of the authors but it should be noted that in certain cases there was not complete unanimity. For the most part, however, it it felt that the accepted names should raise little controversy. The authors will endeavour to use the final suggested namesin future publications, and it is hoped that other investigatorswill find them suitable for their use. -

Development and Achievements of Dutch Northern and Arctic Cartography
ARCTIC’ VOL. 37, NO. 4 (DECEMBER 1984) P. 493.514 Development and Achievements of Dutch Northern and Arctic Cartography. in the Sixteenth and Seventeenth :Centuries GUNTER. SCHILDER* ther north, as far as the Shetlands the Faroes, in line with INTRODUCTION and the expansion of the Dutch .fishing and trading areas. The During the sixteenth and .seventeenth. centuries, the Dutch Thresmr contains a number of coastal viewsfrom the voyage made. a vital contribution to. the mapphg of the northern and around the North Capeas far as ‘‘Wardhuys”. Although there arctic regions, and their caPtographic work piayed a decisive is no mapofthis region, there is.a map of the coasts of Karelia part in expanding. the ,geographical .knowledgeof that time. and Russia to the east of the White Sea asfar as the Pechora, Amsterdam became the centre.of international map production accompanied by a text with instructionsfor navigation as far as and the map trade. Its Cartographers and publishers acquired Vaygach and Novaya Zemlya (Waghenaer, 1592:fo101-105). their knowledge partly from the results of expeditions fitted A coastal view.of the latter is also given.s The fact that Wag- out by theirfellow countrymen and, partlyfrom foreign henaer had access to original sources is shown by the inclusion voyages of discovery. This paper will describe the growing- in the Thresoor of the only known accountof Olivier Brunel’s Dutch..awarenessof .the northern and arctic regions. stage by voyage to-NovayaZemlya in 1584 (Waghenaer, ‘1592:P104).6 stage and region by region, with the aid of Dutch. maps. Anotherimportant document is WillemBiuentsz’s map of northern Scandinavia, which extends as faras the entrance to THE PROGRESS OF DUTCH KNOWLEDGE IN THE NORTH .the White Sea, and shows.al1 the reefs and shallows(Fig. -

Download PDF Van Tekst
Bij noorden om Olivier Brunel en de doorvaart naar China en Cathay in de zestiende eeuw Marijke Spies bron Marijke Spies, Bij noorden om. Amsterdam University Press, Amsterdam 1994 Zie voor verantwoording: http://www.dbnl.org/tekst/spie010bijn01_01/colofon.htm © 2006 dbnl / Marijke Spies *1 Op deze compositiekaart staat aangegeven hoe de noordelijke wereld er volgens de zestiende-eeuwse opvattingen ongeveer uitzag en waar de verschillende plaatsen en rivieren waarvan in Bij noorden om sprake is, werden gesitueerd. In grijze lijnen is onze huidige geografische kennis weergegeven. Op de uitsnede van de zee tussen Noord-Amerika en Scandinavië (links) staan de vele imaginaire eilanden die men daar situeerde. Op de ander uitsnede (rechts) is het gebied tussen de Noordkaap en de Karische Zee, waarlangs de Engelsen en Nederlanders een doortocht zochten, in detail afgebeeld. Marijke Spies, Bij noorden om V But it were to be wished, that none would write Histories with so great a desire of setting foorth novelties and strange things, [...] Arngrimus Ionas Islandus 1592 1 1 Hakluyt 1 1598, p. 561. Marijke Spies, Bij noorden om 1 I De man Zijn naam duikt op uit het niets, verdwijnt, duikt weer op. Vier, vijf vermeldingen, soms met tientallen jaren ertussen, suggereren een levensgeschiedenis die als een rode draad door de noordelijke landen van Europa loopt. Van handelsnederzetting naar koopstad en van haven weer naar handelsnederzetting: een handvol hutten aan de monding van een rivier, gehuchten waarvan de naam sinds lang gewijzigd is of vergeten. Zoveel weten we, dat ergens in het midden van de zestiende eeuw Olivier Brunel, afkomstig uit Brussel of uit Leuven, vanuit Kola aan de noordkust van Lapland naar Kholmogory aan de benedenloop van de Dwina werd gezonden om Russisch te leren.1 Gezonden door wie? Er werd in die tijd nog nauwelijks rond de Noordkaap gevaren (zie afb. -

An Experimental Study of Ice-Bed Separation During Glacial Sliding Benjamin Brett Etp Ersen Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2012 An experimental study of ice-bed separation during glacial sliding Benjamin Brett etP ersen Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Geology Commons, and the Geophysics and Seismology Commons Recommended Citation Petersen, Benjamin Brett, "An experimental study of ice-bed separation during glacial sliding" (2012). Graduate Theses and Dissertations. 12701. https://lib.dr.iastate.edu/etd/12701 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. An experimental study of ice-bed separation during glacial sliding by Benjamin Brett Petersen A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Geology Program of Study Committee: Neal R. Iverson, Major Professor Igor Beresnev Carl E. Jacobson Iowa State University Ames, Iowa 2012 Copyright © Benjamin Brett Petersen, 2012. All rights reserved. ii TABLE OF CONTENTS ACKNOWLEDGMENTS iii ABSTRACT iv CHAPTER 1. INTRODUCTION 1 1.1 Ice-bed separation 1 1.2 Sliding models 2 1.3 Hydrology models 5 1.4 Quarrying 8 1.5 Experimental studies of cavitation 9 1.6 Motivation and objectives 10 CHAPTER 2. METHODS 12 2.1 The ring-shear device 12 2.2 Procedure 19 2.3 Data processing 25 CHAPTER 3. -

Reconstructing the Confluence Zone Between Laurentide and Cordilleran Ice Sheets Along the Rocky Mountain Foothills, Southwest Alberta
This is a repository copy of Reconstructing the confluence zone between Laurentide and Cordilleran ice sheets along the Rocky Mountain Foothills, southwest Alberta. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/105138/ Version: Accepted Version Article: Utting, D., Atkinson, N., Pawley, S. et al. (1 more author) (2016) Reconstructing the confluence zone between Laurentide and Cordilleran ice sheets along the Rocky Mountain Foothills, southwest Alberta. Journal of Quaternary Science, 31 (7). pp. 769-787. ISSN 0267-8179 https://doi.org/10.1002/jqs.2903 Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Reconstructing the confluence zone between Laurentide and Cordilleran ice sheets along the Rocky Mountain Foothills, south-west Alberta Daniel J. Utting1, Nigel Atkinson1, Steven Pawley1, Stephen J. -

Amphibious Hearing in Ringed Seals (Pusa Hispida): Underwater Audiograms, Aerial Audiograms and Critical Ratio Measurements Jillian M
© 2015. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2015) 218, 2250-2259 doi:10.1242/jeb.120972 RESEARCH ARTICLE Amphibious hearing in ringed seals (Pusa hispida): underwater audiograms, aerial audiograms and critical ratio measurements Jillian M. Sills1,*, Brandon L. Southall2,3 and Colleen Reichmuth2 ABSTRACT Measurements of hearing in ringed seals provide information about Ringed seals (Pusa hispida) are semi-aquatic marine mammals with the characteristics of their auditory system and improve a circumpolar Arctic distribution. In this study, we investigate the understanding of their acoustic ecology. Ringed seals are of amphibious hearing capabilities of ringed seals to provide auditory particular interest because of their importance as a subsistence profiles for this species across the full range of hearing. Using resource, their ecological role as primary prey for polar bears (Ursus psychophysical methods with two trained ringed seals, detection maritimus) and their vulnerability to the effects of climate change, thresholds for narrowband signals were measured under quiet, including loss of sea ice and rapid industrialization. carefully controlled environmental conditions to generate aerial and Laboratory studies on hearing have provided information about underwater audiograms. Masked underwater thresholds were the auditory capabilities of some species of phocid (true) seals, but measured in the presence of octave-band noise to determine there is a lack of comprehensive data for many species. The northern critical ratios. Results indicate that ringed seals possess hearing seals (subfamily Phocinae) include the ringed, Baikal (Pusa abilities comparable to those of spotted seals (Phoca largha) and sibirica), Caspian (Pusa caspica), spotted (Phoca largha), harbor harbor seals (Phoca vitulina), and considerably better than (Phoca vitulina), grey (Halichoerus grypus), ribbon (Histriophoca previously reported for ringed and harp seals.