Rhinitis 2020: a Practice Parameter Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Respiratory Syncytial Virus Bronchiolitis in Children DUSTIN K
Respiratory Syncytial Virus Bronchiolitis in Children DUSTIN K. SMITH, DO; SAJEEWANE SEALES, MD, MPH; and CAROL BUDZIK, MD Naval Hospital Jacksonville, Jacksonville, Florida Bronchiolitis is a common lower respiratory tract infection in infants and young children, and respiratory syncytial virus (RSV) is the most common cause of this infection. RSV is transmitted through contact with respiratory droplets either directly from an infected person or self-inoculation by contaminated secretions on surfaces. Patients with RSV bronchiolitis usually present with two to four days of upper respiratory tract symptoms such as fever, rhinorrhea, and congestion, followed by lower respiratory tract symptoms such as increasing cough, wheezing, and increased respira- tory effort. In 2014, the American Academy of Pediatrics updated its clinical practice guideline for diagnosis and man- agement of RSV bronchiolitis to minimize unnecessary diagnostic testing and interventions. Bronchiolitis remains a clinical diagnosis, and diagnostic testing is not routinely recommended. Treatment of RSV infection is mainly sup- portive, and modalities such as bronchodilators, epinephrine, corticosteroids, hypertonic saline, and antibiotics are generally not useful. Evidence supports using supplemental oxygen to maintain adequate oxygen saturation; however, continuous pulse oximetry is no longer required. The other mainstay of therapy is intravenous or nasogastric admin- istration of fluids for infants who cannot maintain their hydration status with oral fluid intake. Educating parents on reducing the risk of infection is one of the most important things a physician can do to help prevent RSV infection, especially early in life. Children at risk of severe lower respiratory tract infection should receive immunoprophy- laxis with palivizumab, a humanized monoclonal antibody, in up to five monthly doses. -

Review of the Existing Recommendations for Essential Medicines for Ear, Nose and Throat Conditions in Adults and Children and Suggested Modifications
REVIEW OF THE EXISTING RECOMMENDATIONS FOR ESSENTIAL MEDICINES FOR EAR, NOSE AND THROAT CONDITIONS IN ADULTS AND CHILDREN AND SUGGESTED MODIFICATIONS 2012 Shelly Chadha, Andnet Kebede Prevention of Blindness and Deafness, World Health Organization REVIEW OF THE EXISTING RECOMMENDATIONS FOR ESSENTIAL MEDICINES (Ear, Nose and Throat conditions) FOR USE IN ADULTS AND CHILDREN AND SUGGESTED MODIFICATIONS Context: The WHO Essential Medicines List includes a section for ENT conditions in children. The current section does not make any reference to medicines and dosages recommended for adults. As most of the conditions for which the listed medicines are indicated, are common in adults, the list needs to be appropriately reviewed in that context. Methodology: Each of the medicines listed in the EML for children was reviewed to consider its appropriateness for inclusion in the EML for adults. The recommended dosages for adults and children were also considered. Recommendations: The medicine list should include a list for adults as well as children. The list for adults should include Xylometazoline hydrochloride nasal spray 0.1%. It should be stated that the medicine (xylometazoline nasal spray) should not be used for prolonged periods of time, unless specifically advised and under medical supervision. The adult list should also include the other medicines mentioned in the list of children, i.e.: o Ciprofloxacin ear drops: 0.3%, as hydrochloride, for tropical use. o Aectic acid ear drops, 2% in alcohol, for topical use. Budesonide nasal spray listed in the EML for children needs greater, in depth review, which may be considered for the next EML update. 1 Xylometazoline Hydrochloride nasal spray Indications: Nasal congestion is obstruction of nasal passages, mainly caused by mucosal inflammation due to increased venous engorgement, nasal secretions and tissue swelling. -

I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St
2014;22(1):13-18 TCP Translational and Clinical Pharmacology http://dx.doi.org/10.12793/tcp.2014.22.1.13 Clinical Pharmacology Review for Primary Health Care Providers: I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul 137-701, Korea *Correspondence: S. Han; Tel: +82-2-2258-7326, Fax: +82-2-2258-7876, E-mail: [email protected] Received 31 May 2014 Primary health care providers play a critical role in maintaining public health, and the appropri- Accepted 31 May 2014 ate use of pharmaceutical products is one of the major parts of their practice. This series of articles, pISSN: 2289-0882 entitled ‘Clinical Pharmacology Review for Primary Health Care Providers,’ is intended to help pri- mary health care providers select more appropriate prescriptions for frequently used drugs based on up-to-date information. We expect that this effort will contribute to improvements in public health and diminish unnecessary drug use. Introduction tion on the H1 receptor.[8] THERAPEU Antihistamines include some of the most frequently prescribed drugs in the primary health care environment for the symp- Generations and Classes tomatic relief of allergic diseases, the common cold, urticaria, Many primary health care providers are well-informed about T and insomnia.[1-5] The importance of antihistamines has been the different ‘generations’ of antihistamines but not about the ICS TU emphasized as the prevalence of target diseases increases.[6,7] different ‘classes’ characterized according to chemical structure. However, the appropriate use, clinical effectiveness, and target [1] This discrepancy seems reasonable because ‘inter-generation’ T populations for prescription of antihistamines are still a matter differences are more prominent than ‘inter-class’ differences. -

Khan CV 9-4-20
CURRICULUM VITAE DAVID A. KHAN, MD University of Texas Southwestern Medical Center 5323 Harry Hines Boulevard Dallas, TX 75390-8859 (214) 648-5659 (work) (214) 648-9102 (fax) [email protected] EDUCATION 1980 -1984 University of Illinois, Champaign IL; B.S. in Chemistry, Magna Cum Laude 1984 -1988 University of Illinois School of Medicine, Chicago IL; M.D. 1988 -1991 Good Samaritan Medical Center, Phoenix AZ; Internal Medicine internship & residency 1991-1994 Mayo Clinic, Rochester MN; Allergy & Immunology fellowship PROFESSIONAL EXPERIENCE 1994 -2001 Assistant Professor of Internal Medicine, UT Southwestern 1997 -1998 Co-Director, Allergy & Immunology Training Program 1998 - Director, Allergy & Immunology Training Program 2002- 2008 Associate Professor of Internal Medicine, UT Southwestern 2008-present Professor of Medicine, UT Southwestern AWARDS/HONORS 1993 Allen & Hanburys Respiratory Institute Allergy Fellowship Award 1993 Von Pirquet Award 2001 Outstanding Teacher 2000-2001 UTSW Class of 2003 2004 Outstanding Teacher 2003-2004 UTSW Class of 2006 2005 Outstanding Teacher 2004-2005 UTSW Class of 2007 2006 Daniel Goodman Lectureship, ACAAI meeting 2007 Most Entertaining Teacher 2006-2007 UTSW Class of 2009 2008 Stanislaus Jaros Lectureship, ACAAI meeting 2009 Outstanding Teacher 2007-2008 UTSW Class of 2010 2011 John L. McGovern Lectureship, ACAAI meeting 2012 I. Leonard Bernstein Lecture, ACAAI meeting 2014 Distinguished Fellow, ACAAI meeting 2015 Elliot F. Ellis Memorial Lectureship, AAAAI meeting 2015 Bernard Berman Lectureship, -

Intranasal Ipratropium Bromide Reduced Rhinorrhea and Improved Cold Symptoms
/:*-..= S Evid Based Med: first published as 10.1136/ebm.1996.1.205 on 1 December 1996. Downloaded from Intranasal ipratropium bromide reduced rhinorrhea and improved cold symptoms Hayden FG, Diamond L, Wood PB, 0.06% in a buffered salt solution (P = 0.003). {This 17% absolute dif- KortsDC, Wecker MT. Effectiveness (2 sprays/nostril [84 u,g] 3 times/d ference in improvement between the and safety of intranasal ipratropium for 4 d) (n = 137), the same nasal ipratropium and placebo groups bromide in common colds. A ran- spray without ipratropium in = 137), means that 6 patients (95% CI, 4 to domized, double-blind, placebo- or no medication (n = 137). No cold 16) would need to be treated with controlled trial. Ann Intern Med. medications other than analgesics ipratropium (rather than placebo) for 1996JullS;12S:89-91. and antitussives were allowed. 4 days to result in improvement for 1 additional patient; the relative risk Main outcome measures improvement was 26%, CI 9% to Objective 47%* }. Rates of nasal dryness (12% To determine whether intxanasal ipra- The main outcome measure was a vs 4%), blood-tinged mucus (17% vs tropium bromide is effective and safe global assessment of overall improve- 4%), and headache (9% vs 2%) were for reducing common cold symptoms. ment (report by patients of being better or much better). Rhinorrhea greater in the ipratropium group than Design was monitored in the clinic hourly in the placebo group. 6-day, randomized, double-blind, for the first 6 hours on day 1 and Conclusion placebo-controlled trial. hourly for 3 hours on day 2. -

Allergic/Non-Allergic Rhinitis
Tips to Remember: Rhinitis Do you have a runny or stuffy nose that doesn't seem to go away? If so, you may have rhinitis, which is an inflammation of the mucous membranes of the nose. Rhinitis is one of the most common allergic conditions in the United States, affecting about 40 million people. It often coexists with other allergic disorders, such as asthma. It is important to treat rhinitis because it can contribute to other conditions such as sleep disorders, fatigue and learning problems. There are two general types of rhinitis: Allergic rhinitis is caused by substances called allergens. Allergens are often common, usually harmless substances that can cause an allergic reaction in some people. Causes • When allergic rhinitis is caused by common outdoor allergens, such as airborne tree, grass and weed pollens or mold, it is called seasonal allergic rhinitis, or "hay fever." • Allergic rhinitis is also triggered by common indoor allergens, such as animal dander (dried skin flakes and saliva), indoor mold or droppings from cockroaches or dust mites. This is called perennial allergic rhinitis. Symptoms • Sneezing • Congestion • Runny nose • Itchiness in the nose, roof of the mouth, throat, eyes and ears Diagnosis If you have symptoms of allergic rhinitis, an allergist/immunologist can help determine which specific allergens are triggering your reaction. He or she will take a thorough health history, and then test you to determine if you have allergies. Skin tests or Blood (RAST) tests are the most common methods for determining your allergic triggers. Treatment Once your allergic triggers are determined, your physician or nurse will work with you to develop a plan to avoid the allergens that trigger your symptoms. -

NUR 6325 Family Primary Care I Fall Semester 2021 Course Information
NUR 6325 Family Primary Care I Fall Semester 2021 Department of Nursing Instructor: Avis Johnson-Smith, DNP, APRN, FNP-BC, CPNP-PC, CPMHS, CNS, FAANP Email: [email protected] Phone: 229-431-2030 Office: Virtual Office Hours: By Appointment. If you have a question and an email response would suffice, then simply let me know this when you contact me. Time Zone: All due dates and times in this syllabus are Central Standard Time (CST) Course Information Course Description The course focus is on the transition from RN to Family Nurse Practitioner in the diagnosis and management of common acute and chronic conditions across the lifespan in a primary care setting. As a provider of family-centered care emphasis is placed on health promotion, risk reduction and evidence- based management of common symptoms and problems. Nursing’s unique contribution to patient care and collaboration with other health care professionals is emphasized. Course Credits Three semester credit hours (3-0-0) This course meets completely online using Blackboard as the delivery method Prerequisite / Co-requisite Courses NUR 6201, 6318, 6323, 6324, 6331, NUR 6327 Prerequisite Skills Accessing internet web sites, use of ASU Library resources, and proficiency with Microsoft Word and/or PowerPoint are expectations of the program name. Computer access requirements are further delineated in the graduate Handbook. Tutorials for ASU Library and for Blackboard are available through RamPort. The ASU Graduate Nursing Student Handbook1 should be reviewed before taking this course. Program Outcomes Upon completion of the program of study for the MSN Program, the graduate will be prepared to: 1. -

Allergic Fungal Airway Disease Rick EM, Woolnough K, Pashley CH, Wardlaw AJ
REVIEWS Allergic Fungal Airway Disease Rick EM, Woolnough K, Pashley CH, Wardlaw AJ Institute for Lung Health, Department of Infection, Immunity & Inflammation, University of Leicester and Department of Respiratory Medicine, University Hospitals of Leicester NHS Trust, Leicester, UK J Investig Allergol Clin Immunol 2016; Vol. 26(6): 344-354 doi: 10.18176/jiaci.0122 Abstract Fungi are ubiquitous and form their own kingdom. Up to 80 genera of fungi have been linked to type I allergic disease, and yet, commercial reagents to test for sensitization are available for relatively few species. In terms of asthma, it is important to distinguish between species unable to grow at body temperature and those that can (thermotolerant) and thereby have the potential to colonize the respiratory tract. The former, which include the commonly studied Alternaria and Cladosporium genera, can act as aeroallergens whose clinical effects are predictably related to exposure levels. In contrast, thermotolerant species, which include fungi from the Candida, Aspergillus, and Penicillium genera, can cause a persistent allergenic stimulus independent of their airborne concentrations. Moreover, their ability to germinate in the airways provides a more diverse allergenic stimulus, and may result in noninvasive infection, which enhances inflammation. The close association between IgE sensitization to thermotolerant filamentous fungi and fixed airflow obstruction, bronchiectasis, and lung fibrosis suggests a much more tissue-damaging process than that seen with aeroallergens. This review provides an overview of fungal allergens and the patterns of clinical disease associated with exposure. It clarifies the various terminologies associated with fungal allergy in asthma and makes the case for a new term (allergic fungal airway disease) to include all people with asthma at risk of developing lung damage as a result of their fungal allergy. -

Acute Allergic Rhinitis
South African Family Practice ISSN: (Online) 2078-6204, (Print) 2078-6190 Page 1 of 6 Open Forum Acute allergic rhinitis Authors: Allergic rhinitis is a common and troubling condition. Basic management of this condition has 1 Robin J. Green been well described. However, acute exacerbations of the chronic condition allergic rhinitis Andre van Niekerk1,2 Marinda McDonald3 are a seldom discussed or described problem despite the fact that even well-controlled patients Raymond Friedman4 frequently have exacerbations. This consideration means that a new approach is necessary to Charles Feldman5 define the management of these patients. There are three important events that illustrate the 5 Guy Richards need for a new therapeutic approach: Fatima Mustafa1 Affiliations: • A person who gets a new diagnosis of allergic rhinitis, but has symptoms for many months 1Department of Paediatrics or years and Child Health, University • A sufferer of allergic rhinitis who is exposed to an environment that triggers an exacerbation of Pretoria, Pretoria, • A person who has an exacerbation related to another trigger. South Africa 2Private Practice, Netcare Recognition of triggers and management strategies to correctly use ‘relief’ therapies such as Clinton Clinic, Alberton, topical nasal decongestants is the key to successful management. In addition, the use of an South Africa ‘action plan’, as for asthma, is useful. 3Private Practice, Blairgowrie, Keywords: allergic rhinitis; acute exacerbations; triggers; allergens; topical decongestants; Johannesburg, South Africa intranasal steroids. 4Private Practice, Mediclinic Sandton, Johannesburg, South Africa Introduction Allergic rhinitis (AR) is a common inflammatory process that manifests as nasal itchiness and 5Department of Medicine, University of the congestion, postnasal drip, sore throat, cough, itchy swollen eyes and sometimes airway hyper- Witwatersrand, reactivity (AHR) if asthma coexists. -

FDA Warns That Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm This Includes Heart and Mental Health Problems Or Death
FDA warns that abuse and misuse of the nasal decongestant propylhexedrine causes serious harm This includes heart and mental health problems or death 3-25-2021 FDA Drug Safety Communication What safety concern is FDA announcing? The U.S. Food and Drug Administration (FDA) is warning that the abuse and misuse of the over- the-counter (OTC) nasal decongestant propylhexedrine can lead to serious harm such as heart and mental health problems. Some of these complications, which include fast or abnormal heart rhythm, high blood pressure, and paranoia, can lead to hospitalization, disability, or death. Reports of individuals abusing and misusing propylhexedrine have increased in recent years. Propylhexedrine is safe and effective when used as directed. What is FDA doing? We are requesting that all manufacturers of OTC propylhexedrine nasal decongestant inhalers consider product design changes that support its safe use. For example, modifying the product to create a physical barrier that would make tampering with the device and abusing the propylhexedrine inside more difficult. In addition, decreasing the amount of medicine the device contains could also reduce the risk of serious side effects if abused or misused. We continue to evaluate this safety issue and will determine if additional FDA actions are needed. What is propylhexedrine and how can it help me? Propylhexedrine is a nasal decongestant that is available OTC in an inhaler. It is used short term to temporarily relieve nasal congestion due to colds, hay fever, or other upper respiratory allergies. It works by reducing swelling and inflammation of the mucous membrane lining of the nose. -
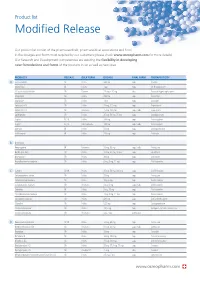
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Sudafed Decongestant Tablets 12S PIL SNAS 3359 SNAS 3557 14 Mm 29.07.2021 English Rate and Blood Pressure)
12 mm 12 mm spot = 12 x 2 centered in Distance between cutted the margin of 14 mm line/middel of the spot = 51.6 mm UK_Sudafed Decongestant Tablets 12s_PIL_SNAS 3359_SNAS 3557 14 mm 29.07.2021 English rate and blood pressure). 402059927_r2 N/A Now read this whole leaflet carefully before you use this medicine. Keep the leaflet: you ■ If you have an overactive thyroid Brochure/Leaet/Insert 145 x 250 mm might need it again. gland. 360165B BL-Val de Reuil_France ■ If you have glaucoma (increased CHILD 360165A Multi Packaging Solutions St Pierre 1 What the medicine is for pressure in the eye). 953 Oset Printing Sudafed Decongestant Tablets is a medicine used ■ If you have severe kidney problems. 8169503 PAPER to provide relief from cold, flu and allergy ■ If you are taking beta blockers (used to EMEA_2020_00006767_001 PC-0002778 symptoms such as blocked sinuses, stuffy nose treat high blood pressure). and catarrh. ■ If you are taking, or have taken in the The tablets contain pseudoephedrine last two weeks, drugs for depression ■ This medicine is used to help clear cold, flu hydrochloride, which is a decongestant that known as Monoamine Oxidase and allergy symptoms such as blocked relieves nasal and sinus congestion. Inhibitors (MAOIs) or Reversible Black PANTONE sinuses, stuffy nose and catarrh. This medicine is for use by adults and children Inhibitors of Monoamine Oxidase 3005 C ■ This medicine is for use by adults and (RIMAs). LITHO OFFSET LITHO OFFSET aged 12 years and over. children aged 12 years and over. 145 mm REPRESENTATION: Colours represented with a diagonal line have been modified to aid PDF approval.