Intranasal Ipratropium Bromide Reduced Rhinorrhea and Improved Cold Symptoms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Respiratory Syncytial Virus Bronchiolitis in Children DUSTIN K
Respiratory Syncytial Virus Bronchiolitis in Children DUSTIN K. SMITH, DO; SAJEEWANE SEALES, MD, MPH; and CAROL BUDZIK, MD Naval Hospital Jacksonville, Jacksonville, Florida Bronchiolitis is a common lower respiratory tract infection in infants and young children, and respiratory syncytial virus (RSV) is the most common cause of this infection. RSV is transmitted through contact with respiratory droplets either directly from an infected person or self-inoculation by contaminated secretions on surfaces. Patients with RSV bronchiolitis usually present with two to four days of upper respiratory tract symptoms such as fever, rhinorrhea, and congestion, followed by lower respiratory tract symptoms such as increasing cough, wheezing, and increased respira- tory effort. In 2014, the American Academy of Pediatrics updated its clinical practice guideline for diagnosis and man- agement of RSV bronchiolitis to minimize unnecessary diagnostic testing and interventions. Bronchiolitis remains a clinical diagnosis, and diagnostic testing is not routinely recommended. Treatment of RSV infection is mainly sup- portive, and modalities such as bronchodilators, epinephrine, corticosteroids, hypertonic saline, and antibiotics are generally not useful. Evidence supports using supplemental oxygen to maintain adequate oxygen saturation; however, continuous pulse oximetry is no longer required. The other mainstay of therapy is intravenous or nasogastric admin- istration of fluids for infants who cannot maintain their hydration status with oral fluid intake. Educating parents on reducing the risk of infection is one of the most important things a physician can do to help prevent RSV infection, especially early in life. Children at risk of severe lower respiratory tract infection should receive immunoprophy- laxis with palivizumab, a humanized monoclonal antibody, in up to five monthly doses. -

Allergic Fungal Airway Disease Rick EM, Woolnough K, Pashley CH, Wardlaw AJ
REVIEWS Allergic Fungal Airway Disease Rick EM, Woolnough K, Pashley CH, Wardlaw AJ Institute for Lung Health, Department of Infection, Immunity & Inflammation, University of Leicester and Department of Respiratory Medicine, University Hospitals of Leicester NHS Trust, Leicester, UK J Investig Allergol Clin Immunol 2016; Vol. 26(6): 344-354 doi: 10.18176/jiaci.0122 Abstract Fungi are ubiquitous and form their own kingdom. Up to 80 genera of fungi have been linked to type I allergic disease, and yet, commercial reagents to test for sensitization are available for relatively few species. In terms of asthma, it is important to distinguish between species unable to grow at body temperature and those that can (thermotolerant) and thereby have the potential to colonize the respiratory tract. The former, which include the commonly studied Alternaria and Cladosporium genera, can act as aeroallergens whose clinical effects are predictably related to exposure levels. In contrast, thermotolerant species, which include fungi from the Candida, Aspergillus, and Penicillium genera, can cause a persistent allergenic stimulus independent of their airborne concentrations. Moreover, their ability to germinate in the airways provides a more diverse allergenic stimulus, and may result in noninvasive infection, which enhances inflammation. The close association between IgE sensitization to thermotolerant filamentous fungi and fixed airflow obstruction, bronchiectasis, and lung fibrosis suggests a much more tissue-damaging process than that seen with aeroallergens. This review provides an overview of fungal allergens and the patterns of clinical disease associated with exposure. It clarifies the various terminologies associated with fungal allergy in asthma and makes the case for a new term (allergic fungal airway disease) to include all people with asthma at risk of developing lung damage as a result of their fungal allergy. -

Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: 3 Prevalence, Severity, Timing and Associated Characteristics 4 5 Marlene M
Complete Manuscript Click here to access/download;Complete Manuscript;manuscript 042220 v3.docx This manuscript has been accepted for publication in Otolaryngology-Head and Neck Surgery. 2 Olfactory dysfunction and sinonasal symptomatology in COVID-19: 3 prevalence, severity, timing and associated characteristics 4 5 Marlene M. Speth, MD, MA1, Thirza Singer-Cornelius, MD1, Michael Obere, PhD2, Isabelle 6 Gengler, MD3, Steffi J. Brockmeier, MD1, Ahmad R. Sedaghat, MD, PhD3 7 8 9 1Klinik für Hals-, Nasen-, Ohren- Krankheiten, Hals-und Gesichtschirurgie, Kantonsspital 10 Aarau, Switzerland, 2Institute for Laboratory Medicine, Kantonsspital Aarau, Aarau, 11 Switzerland, 3Department of Otolaryngology—Head and Neck Surgery, University of 12 Cincinnati College of Medicine, Cincinnati, OH, USA. 13 14 15 Funding: MMS and TSC received funding from Kantonsspital Aarau, Department of 16 Otolaryngology, Funded by Research Council KSA 1410.000.128 17 18 Conflicts of Interest: None 19 20 21 Authors’ contributions: 22 MMS: designed and performed study, wrote and revised manuscript, approved final 23 manuscript. 24 TSC: designed and performed study, approved final manuscript. 25 MO: performed study, approved final manuscript. 26 IG: designed study, revised manuscript and approved final manuscript 27 SJB: designed and performed study, revised manuscript and approved final manuscript 28 ARS: conceived, designed and performed study, wrote and revised manuscript, approved 29 final manuscript. 30 31 32 Corresponding Author: 33 Ahmad R. Sedaghat, MD, PhD 34 -

Global Strategy for Asthma Management and Prevention, 2019. Available From
DISTRIBUTE OR COPY NOT DO MATERIAL- COPYRIGHTED ASTHMA MANAGEMENT AND PREVENTION GLOBAL STRATEGY FOR Updated 2019 9 Global Strategy for Asthma Management and Prevention (2019 update) DISTRIBUTE OR COPY NOT DO The reader acknowledges that this reportMATERIAL- is intended as an evidence-based asthma management strategy, for the use of health professionals and policy-makers. It is based, to the best of our knowledge, on current best evidence and medical knowledge and practice at the date of publication. When assessing and treating patients, health professionals are strongly advised to use their own professional judgment, and to take into account local or national regulations and guidelines. GINA cannot be held liable or responsible for inappropriate healthcare associated with the use of this document, including any use which is not in accordance with applicable local or national regulations or COPYRIGHTEDguidelines. This document should be cited as: Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2019. Available from: www.ginasthma.org 1 Table of contents Tables and figures ............................................................................................................................................................... 5 Preface ................................................................................................................................................................................. 7 Members of GINA committees (2018) ................................................................................................................................ -

Snotty Noses – When Is It Sinusitis? Objectives
11/2/2019 Snotty Noses – When is it Sinusitis? Uma Ramaswamy, MD Objectives • Identify and medically manage acute rhinosinusitis • Recognize signs/symptoms of pediatric chronic rhinosinusitis (PCRS) • Recognize refractory or complicated sinusitis • When to refer to ENT 1 11/2/2019 https://www.sciencephoto.com/media/728470/view https://www.sciencephoto.com/media/728470/view 2 11/2/2019 Physiology and Function • Humidification • Temperature modification • Filtration of inspired air • Olfaction https://openi.nlm.nih.gov/imgs/512/28/2719610/PMC2719610_1465-9921-10-61-2.png https://hosting.med.upenn.edu/otocme/wp-content/uploads/sites/25/2016/12/pci4-300x183.jpg Pediatric Rhinosinusitis • Inflammation of the nasal cavity and paranasal sinuses Two or more symptoms • Nasal obstruction/congestion • Nasal Discharge – Anterior – Rhinorrhea – Posterior – Post-nasal drip (PND) • Cough • Facial Pain or Pressure • Fever 3 11/2/2019 Contributing Factors • Anatomic Factors • Infectious Agents – Bacterial versus Viral • Biofilms • Adenoids • Allergic Rhinitis • Immunodeficiency • Primary Ciliary Dyskinesia • Cystic Fibrosis Differential Diagnosis of Pediatric Sinusitis • Allergic rhinitis • Chronic invasive fungal sinusitis • Cystic fibrosis • Sinonasal neoplasm • Immotile cilia • Upper respiratory tract infection syndrome/Primary ciliary dyskinesia • Unilateral choanal atresia – Kartagener syndrome (situs • Adenoid hypertrophy inversus, chronic sinusitis, and bronchiectasis) • Nasal foreign body • Odontogenic • Immunodeficiency (immunoglobulin [Ig]A, -

Respiratory Syncytial Virus Infection and Bronchiolitis
Respiratory Syncytial Virus Infection and Bronchiolitis Giovanni Piedimonte, MD,*† Miriam K. Perez, MD*‡ *Cleveland Clinic Pediatric Institute, Cleveland, OH. †Cleveland Clinic Children’s Hospital for Rehabilitation, The Cleveland Clinic, Cleveland, OH. ‡Department of Community Pediatrics, Independence Family Health Center, Independence, OH. Practice Gaps 1. Respiratory syncytial virus (RSV) is the most common respiratory pathogen in infants and young children worldwide. Although the most effective management of this infection remains supportive care, many patients continue to be managed with therapies that lack the support of scientific evidence. 2. Although the quest for a safe and effective vaccine remains unsuccessful, the more vulnerable patients can be protected with passive prophylaxis. Because of limited clinical benefits and high costs, RSV prophylaxis should be limited to high-risk infants as directed by the most current evidence-based guidelines that, however, are not consistently followed. 3. The acute phase of this infection is often followed by episodes of wheezing that recur for months or years and usually lead to a physician diagnosis of asthma. The phenotype of post-RSV wheezing is different from atopic asthma, yet it is usually managed using the same pharmacologic therapy with often ineffective results. Objectives After reading this article, readers should be able to: 1. Understand the microbiology, epidemiology, pathophysiology, and AUTHOR DISCLOSURE Dr Perez has disclosed clinical manifestations of RSV bronchiolitis in infants and children. fi no nancial relationships relevant to this fi article. Dr Piedimonte has disclosed he 2. Know the scienti c evidence relevant to prophylactic and therapeutic receives research grant NHLBI HL-061007 strategies currently available and recognize the lack of evidence from the National Heart, Lung and Blood concerning several pharmacologic agents commonly used in the Institute of the National Institutes of Health. -

An Uncommon Etiological Factor for Aspiration Pneumonitis Caused By
Cao et al. BMC Pulm Med (2021) 21:254 https://doi.org/10.1186/s12890-021-01620-5 CASE REPORT Open Access An uncommon etiological factor for aspiration pneumonitis caused by spontaneous sphenoid sinus meningoencephalocele with cerebrospinal fuid rhinorrhea: a case report Jiayu Cao1†, Wei Liu2†, Li Wang1, Yujuan Yang1, Yu Zhang1* and Xicheng Song1 Abstract Background: Aspiration pneumonitis is an infammatory disease of the lungs which is difcult to diagnose accu- rately. Large-volume aspiration of oropharyngeal or gastric contents is essential for the development of aspiration pneumonitis. The role of cerebrospinal fuid (CSF) rhinorrhea is often underestimated as a rare etiological factor for aspiration in the diagnosis process of aspiration pneumonitis. Case presentation: We present a case of a patient with 4 weeks of right-sided watery rhinorrhea accompanied by intermittent postnasal drip and dry cough as the main symptoms. Combined with clinical symptoms, imaging exami- nation of the sinuses, and laboratory examination of nasal secretions, she was initially diagnosed as spontaneous sphenoid sinus meningoencephalocele with CSF rhinorrhea, and intraoperative endoscopic fndings and postopera- tive pathology also confrmed this diagnosis. Her chest computed tomography showed multiple focculent ground glass density shadows in both lungs on admission. The patient underwent endoscopic resection of meningoenceph- alocele and repair of skull base defect after she was ruled out of viral pneumonitis. Symptoms of rhinorrhea and dry cough disappeared, and pneumonitis was improved 1 week after surgery and cured 2 months after surgery. Persistent CSF rhinorrhea caused by spontaneous sphenoid sinus meningoencephalocele was eventually found to be a major etiology for aspiration pneumonitis although the absence of typical symptoms and well-defned risk factors for aspira- tion, such as dysphagia, impaired cough refex and refux diseases. -

Diagnosis and Treatment of Respiratory Illness in Children and Adults Non-Infectious Rhinitis Algorithm
Health Care Guideline: Diagnosis and Treatment of Respiratory Illness in Children and Adults Non-Infectious Rhinitis Algorithm Patient presents with symptoms of non-infectious rhinitis History/physical Consider RAST* and skin yes testing when definitive Signs and symptoms no Signs and symptoms Consider referral to diagnosis is needed suggest allergic suggest structural specialist etiology? etiology? yes no * Radioallergosorbent test Treatment options Non-allergic • Education on avoidance rhinitis • Medications - Intranasal corticosteroids - Intranasal antihistamines - Oral antihistamines Treatment options - Combination intranasal • Medications antihistamines/intranasal corticosteroids - Intranasal antihistamines - Leukotriene blockers - Decongestants - Anticholinergics - Intranasal corticosteroids - Decongestants - Intranasal ipraptropium bromide • Patient education Adequate yes • Patient education Adequate yes • Follow-up as response? • Follow-up as appropriate response? appropriate no no Consider referral • Consider testing to a specialist • Consider referral to a specialist www.icsi.org Copyright © 2017 by Institute for Clinical Systems Improvement 1 Diagnosis and Treatment of Respiratory Illness in Children and Adults Fifth Edition/September 2017 Acute Pharyngitis Algorithm Patient presents with symptoms of GAS* pharyngitis History/physical Shared decision-making Consider strep testing Do not routinely test if Centor (RADT**, throat culture, criteria < 3 or when viral features PCR***) based on clinical like rhinorrhea, cough, oral -
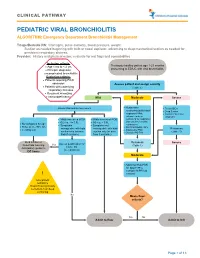
PEDIATRIC VIRAL BRONCHIOLITIS ALGORITHM: Emergency Department Bronchiolitis Management
CLINICAL PATHWAY PEDIATRIC VIRAL BRONCHIOLITIS ALGORITHM: Emergency Department Bronchiolitis Management Triage/Bedside RN: Vital signs, pulse oximetry, blood pressure, weight. Suction as needed beginning with bulb or nasal aspirator, advancing to deep/mechanical suction as needed for persistent respiratory distress. Provider: History and physical exam, evaluate for red flags and comorbidities Inclusion criteria: • Age 1 mo to < 2 yrs Previously healthy patient age 1-23 months • Principle diagnosis: presenting to ED/UC with viral bronchiolitis uncomplicated bronchiolitis Exclusion criteria: • Patients requiring PICU admission Assess patient and assign severity • Patients with underlying (Table 1) respiratory illnesses • Recurrent wheezing • Immunodeficiency Mild Moderate Severe • Assess WOB and O2 requirement Noninvasive • Treat ABCs suctioning (bulb/nasal • Deep Suction aspirator) PRN; • Consider alternative advance to deep diagnosis • Mildly increased WOB • Mildly increased WOB suctioning for respiratory • O2 req. </= 0.5L • O2 req. > 0.5L distress unrelieved by • No tachypnea for age • Symptoms • Symptoms not noninvasive • Pulse ox >/= 90% RA • manageable with bulb manageable with bulb O2 PRN if SpO2 <88% Reassess • Feeding well • Antipyretics PRN suction only (or nose suction only (or nose • Consider PO Trial (Table 1) frida if available) frida if available) Bulb suction or Reassess Assess qualification for Severe NoseFrida teaching Yes (Table 1) Home O2 Anticipatory guidance (see protocol) DC home Moderate • Adjust O2 flow PRN for -

Outpatient Acute Bronchitis Treatment Algroithm for Adults
Acute Bronchitis for Adult and Pediatric Patients Algorithm Strategies to reduce antibiotic use for acute bronchitis: Symptoms consistent with acute bronchitis lasting > 5 days: 1. Use delayed prescription Conduct Differential Diagnosis: Cough, sputum production, strategies Pneumonia, asthma, exacerbation of dyspnea, nasal congestion, COPD, heart failure, upper respiratory 2. Discuss the expected course headache, and fever tract infection of illness and cough duration (2-3 weeks) Symptoms are not consistent 3. Explain that the illness is Acute bronchitis is likely either with acute bronchitis typically caused by a virus viral or Mycoplasma or (Bronchitis is ruled out as a (90%) and not bacteria Chlamydia pneumonia diagnosis) 4. Explain that antibiotics do not significantly shorten illness Are the following Antibiotics are not duration and are associated Are the following symptoms present: indicated for treatment. with adverse effects and symptoms present: Dyspnea, bloody or rusty antibiotic resistance malaise, rhinorrhea, mild sputum, pulse > 100 or paroxysmal cough, bpm, RR > 24 bpm, File TM. Acute bronchitis in Adults. In: excessive lacrimation, T > 100°F (37.8°C), Focal UpToDate, Bond S, Aronson MD (Ed), conjunctival infection? UpToDate, Waltham, MA. (Accessed on consolidation, egophony, April 13, 2017.) Kinkade S, Long NA. Acute or fremitus on chest Bronchitis. Am Fam Physician. Yes 2016;94(7):560-565. Lexicomp Online® , examination, delirium if Dosing: Adult and Pediatric, Hudson, Ohio: age > 75? Lexi-Comp, Inc.; April 13, 2017. Yes, pertussis is likely No Supportive care and symptom management Chest radiography • Antitussives: Dextromethorphan, is indicated Adult: guaifenesin (adults only), 1. Azithromycin 500 mg x1 day, then benzonatate (Rx only) 250 mg x4 • Expectorants: Guaifenesin Infiltrate No Infiltrate 2. -

Current Concepts in Adult Acute Rhinosinusitis ANN M
Current Concepts in Adult Acute Rhinosinusitis ANN M. ARING, MD, and MIRIAM M. CHAN, PharmD, OhioHealth Riverside Methodist Hospital, Columbus, Ohio Acute rhinosinusitis is one of the most common conditions that physicians treat in ambulatory care. Most cases of acute rhinosinusitis are caused by viral upper respiratory infections. A meta-analysis based on individual patient data found that common clinical signs and symptoms were not effective for identifying patients with rhinosinusitis who would benefit from antibiotics.C-reactive protein and erythrocyte sedimentation rate are somewhat useful tests for confirming acute bacterial maxillary sinusitis. Four signs and symptoms that significantly increase the likelihood of a bacterial cause when present are double sickening, purulent rhinorrhea, erythrocyte sedimentation rate greater than 10 mm per hour, and purulent secretion in the nasal cavity. Although cutoffs vary depending on the guideline, anti- biotic therapy should be considered when rhinosinusitis symptoms fail to improve within seven to 10 days or if they worsen at any time. First-line antibiotics include amoxicillin with or without clavulanate. Current guidelines support watchful waiting within the first seven to 10 days after upper respiratory symptoms first appear. Evidence on the use of analgesics, intranasal corticosteroids, and saline nasal irrigation for the treatment of acute rhinosinusitis is poor. Nonetheless, these therapies may be used to treat symptoms within the first 10 days of upper respiratory infection. Radiography is not recommended in the evaluation of uncomplicated acute rhinosinusitis. For patients who do not respond to treatment, computed tomography of the sinuses without contrast media is helpful to evaluate for possible complications or anatomic abnormalities. -

Runny Nose When Is It a Sign of a More Serious Medical Condition?
SPRING 2012 Rhinorrhea: The medical term for A Runny Nose When is it a sign of a more serious medical condition? B. Todd Schaeffer, M.D., F.A.C.S. Lake Success, NY Having a “runny nose “ is very common and is frequently associated with a cold, virus, allergy or sinus infection. Thirty-seven million Americans suffer with chronic sinusitis and fifty million have some form of respiratory allergies. Recently, Cathy, a CSF Rhinorrhea forty-three year old over weight diabetic woman called her primary care doctor (PCP) with a new Cerebrospinal Fluid (CSF) is a clear problem. Her nose started to “run” without fluid produced by the choroid sustaining any trauma. She asked what should she do? plexus in the ventricles of the brain. She denied any symptoms of facial pressure, It acts as a shock absorber and headache, fever, postnasal drip or congestion. Her cushions the brain and spine. The CSF circulates around them in the PCP thought it sounded like allergies and told her to sub-arachnoid space. A start the over the counter anti-histamine Loratidine. communication with this space Cathy had some health issues which were controlled through the arachnoid (thin layer), but had no history of sinusitis, asthma or allergies. dura (thick fibrous layer) and a bony She did note some increased blurring of vision but defect at the skull base, into the attributed this to requiring stronger glasses. After a paranasal sinuses, leads to a leakage few days the nasal discharge worsened especially of clear fluid from one side of the when she leaned forward (Figure #2).