Peer-Review Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Oh Ho Oh Oh Oh Oh O Ho Ch3o N N H O Ho Oh Oh Oh Oh O Ho Ch3o N N
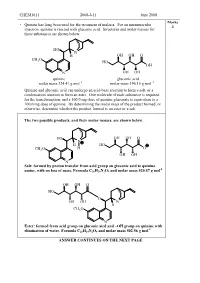
CHEM1611 2008-J-11 June 2008 Marks • Quinine has long been used for the treatment of malaria. For an intramuscular 4 injection, quinine is reacted with gluconic acid. Structures and molar masses for these substances are shown below. HO N H OH OH O CH O 3 HO OH N OH OH quinine gluconic acid molar mass 324.41 g mol –1 molar mass 196.16 g mol –1 Quinine and gluconic acid can undergo an acid-base reaction to form a salt, or a condensation reaction to form an ester. One molecule of each substance is required for the transformation, and a 160.0 mg dose of quinine gluconate is equivalent to a 100.0 mg dose of quinine. By determining the molar mass of the product formed, or otherwise, determine whether the product formed is an ester or a salt. The two possible products, and their molar masses, are shown below. HO OH OH O N H HO CH3O H O OH OH N Salt: formed by proton transfer from acid group on gluconic acid to quinine -1 amine, with no loss of mass. Formula C 26 H26 N2O9 and molar mass 520.57 g mol OH OH O HO O OH OH N H CH3O N Ester: formed from acid group on gluconic aicd and –OH group on quinine with -1 elimination of water. Formula C 26 H24 N2O8 and molar mass 502.56 g mol ANSWER CONTINUES ON THE NEXT PAGE CHEM1611 2008-J-11 June 2008 100.0 mg of quinine corresponds to: ̡̭̳̳ ̊̉̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.083 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̌̋̍.̍̊ ̧ ̭̯̬ ̊ 160.0 mg of the salt product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.074 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̋̉.̎̐ ̧ ̭̯̬ ̊ 160.0 mg of the ester product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.184 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̉̋.̎̏ ̧ ̭̯̬ ̊ As the dosages are the same, it must be the salt which is being administered. -

Genomewide and Enzymatic Analysis Reveals Efficient D-Galacturonic
RESEARCH ARTICLE Molecular Biology and Physiology Genomewide and Enzymatic Analysis Reveals Efficient D-Galacturonic Acid Metabolism in the Basidiomycete Yeast Rhodosporidium toruloides Ryan J. Protzko,a,b Christina A. Hach,c Samuel T. Coradetti,b,d Magdalena A. Hackhofer,c Sonja Magosch,c Nils Thieme,c Gina M. Geiselman,b,d Adam P. Arkin,b,d,f Jeffrey M. Skerker,b,d,f John E. Dueber,b,e,f J. Philipp Benzc aDepartment of Molecular and Cell Biology, University of California, Berkeley, California, USA bEnergy Biosciences Institute, Berkeley, California, USA cHolzforschung München, TUM School of Life Sciences Weihenstephan, Technische Universität München, Freising, Germany dEnvironmental Genomics and Systems Biology Division, Lawrence Berkeley National Laboratory, Berkeley, California, USA eBiological Systems & Engineering Division, Lawrence Berkeley National Laboratory, Berkeley, California, USA fDepartment of Bioengineering, University of California, Berkeley, California, USA ABSTRACT Biorefining of renewable feedstocks is one of the most promising routes to replace fossil-based products. Since many common fermentation hosts, such as Saccharomyces cerevisiae, are naturally unable to convert many component plant cell wall polysaccharides, the identification of organisms with broad catabolism capabilities represents an opportunity to expand the range of substrates used in fer- mentation biorefinery approaches. The red basidiomycete yeast Rhodosporidium to- ruloides is a promising and robust host for lipid- and terpene-derived chemicals. Pre- vious studies demonstrated assimilation of a range of substrates, from C5/C6 sugars to aromatic molecules similar to lignin monomers. In the current study, we analyzed the potential of R. toruloides to assimilate D-galacturonic acid, a major sugar in many pectin-rich agricultural waste streams, including sugar beet pulp and citrus peels. -

Xylooligosaccharides Production, Quantification, and Characterization
19 Xylooligosaccharides Production, Quantification, and Characterization in Context of Lignocellulosic Biomass Pretreatment Qing Qing1, Hongjia Li2,3,4,Ã, Rajeev Kumar2,4 and Charles E. Wyman2,3,4 1 Pharmaceutical Engineering & Life Science, Changzhou University, Changzhou, China 2 Center for Environmental Research and Technology, University of California, Riverside, USA 3 Department of Chemical and Environmental Engineering, University of California, Riverside, USA 4 BioEnergy Science Center, Oak Ridge, USA 19.1 Introduction 19.1.1 Definition of Oligosaccharides Oligosaccharides, also termed sugar oligomers, refer to short-chain polymers of monosaccharide units con- nected by a and/or b glycosidic bonds. In structure, oligosaccharides represent a class of carbohydrates between polysaccharides and monosaccharides, but the range of degree of polymerization (DP, chain length) spanned by oligosaccharides has not been consistently defined. For example, the Medical Subject Headings (MeSH) database of the US National Library of Medicine defines oligosaccharides as carbohy- drates consisting of 2–10 monosaccharide units; in other literature, sugar polymers with DPs of up to 30–40 have been included as oligosaccharides [1–3]. ÃPresent address: DuPont Industrial Biosciences, Palo Alto, USA Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals, First Edition. Edited by Charles E. Wyman. Ó 2013 John Wiley & Sons, Ltd. Published 2013 by John Wiley & Sons, Ltd. 392 Aqueous Pretreatment of Plant Biomass for -

Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacte
S. Dinç et al. / Hacettepe J. Biol. & Chem., 2017, 45 (4), 603-607 Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacter Suşunun Glukonik Asit Üretiminde Kullanımı Research Article Saliha Dinç 1,2*, Meryem Kara2,3, Mehmet Öğüt3, Fatih Er1, Hacer Çiçekçi2 1Selcuk University Cumra School of Applied Sciences, Konya-Turkey. 2Selcuk Uni. Advanced Technology Research and Application Center, Konya-Turkey. 3Selcuk University Cumra Vocational High School, Konya-Turkey. ABSTRACT luconic acid, a food additive, is used in many foods to control acidity or binds metals such as calcium, Giron. Acinetobacter sp. WR326, newly isolated from soil possesses high phosphate solubilizing activity and do not require pyrroloquinoline quinone (PQQ) for glucose dehydrogenase (GDH) activity as cofactor In this study the gluconic acid production potential of this bacterium was investigated. Firstly, Acinetobacter sp. WR326 was incubated in tricalcium phosphate medium (TCP) with varying glucose concentrations (100, 250, 500 mM), at a temperature of 30°C for 5 days (120 hours). The highest gluconic acid yield (59%) was found at a glucose concentration of 100 mM. Then three different levels of gluconic acid addition to the medium (50, 100, 200 mM) were tested. When Acinetobacter sp. WR326 strain was cultivated with a 100 mM glucose and 100 mM gluconic acid the yield increased to 95.27%. In any trials 2 -keto D-gluconic acid, causes problems in processing and purification of the gluconic acid, was not detected in the medium. As a conclusion, Acinetobac- ter sp. WR326 may be considered novel potential bacterial strain for gluconic acid production. -

The Possibility of Recovering of Hydroxytyrosol from Olive Milling Wastewater by Enzymatic Bioconversion 265
ProvisionalChapter chapter 14 The PossibilityPossibility of of Recovering Recovering of Hydroxytyrosolof Hydroxytyrosol from fromOlive Milling Wastewater by Enzymatic Bioconversion Olive Milling Wastewater by Enzymatic Bioconversion Manel Hamza and Sami Sayadi Manel Hamza and Sami Sayadi Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/64774 Abstract This chapter discusses an innovative approach to obtain liquid fractions from olive mill wastewater (OMW) rich in hydroxytyrosol. The method is based on bioconversion combined with membrane separation techniques. An enzymatic bioconversion of three types of OMW was tested. TheS total volumes of OMW are 15 and 40 L. The reaction was monitored in mechanically stirred systems for 2 h at 50°C. Maximum hydroxytyr‐ osol concentrations of about 1.53, 0.83 and 0.46 g/L in the presence of 5 IU Aspergillus niger β‐glucosidase per milliliter from North OMW and South OMW were procured by two different olive millings, which are milling super press (MSP) and milling continuous chain (MCC), respectively. Enzymatic pretreatment was followed by two tangential flow membrane separation stages, microfiltration (MF) and ultrafiltration (UF). The ultrafiltration permeate was concentrated by evaporation at 45°C for 2 h. The latter exhibited a chemical oxygen demand (COD) level of 48.44 g/L. The UF permeate dehydration increased the hydroxytyrosol concentration to 7.2 g/L. A new natural product that contains some minerals beneficial to health and devoid of heavy metals or chemicals was obtained by this innovative work which describes an environmentally friendly process at pilot‐scale. -

4202-B: Nucleic Acids and Carbohydrates L-4 1 Deoxy Sugars
4202-B: Nucleic acids and Carbohydrates L-4 Deoxy sugars In these sugars one of the OH groups is replaced by a hydrogen. 2-Deoxyribose (oxygen missing at C-2 position) is an important example of a deoxy sugar. It is important component of DNA, and lack of C-2 hydroxyl provide additional stability to it as compared to RNA as no intramolecular nuclephilic attack on phosphate chain can occur. Amino sugars In amino sugars one of the OH groups is replaced by an amino group. These molecules allow proteins and sugars to combine and produce structures of remarkable variety and beauty. The most common amino sugars are N-acetyl glucosamine and N-acetyl galactosamine, which differ only in stereochemistry. The hard outer skeletons of insects and crustaceans contain chitin, a polymer very like cellulose but made of N-acetyl glucosamine instead of glucose itself. It coils up in a similar way and provides the toughness of crab shells and beetle cases. Some important antibiotics contain amino sugars. For example, the three subunits of the antibiotic gentamicin are deoxyamino sugars (the middle subunit is missing the ring oxygen). N-Acetyl glucosamine N-Acetyl galactosamine Gentamicin, an antibiotic Cell membranes must not be so impermeable as they need to allow the passage of water and complex molecules. These membranes contain glycoproteins—proteins with amino sugar residues attached to asparagine, serine, or threonine in the protein. The attachment is at the anomeric position so that these compounds are O- or N-glycosides of the amino sugars. The structure below shows N-acetyl galactosamine attached to an asparagine residue as an N-glycoside. -

Wöhler Synthesis of Urea
Wöhler synthesis of urea Wöhler, 1928 – – + + NH Cl + K N C O 4 NH4 N C O O H2N NH2 Friedrich Wöhler Annalen der Physik und Chemie 1828, 88, 253–256 Significance: Wöhler was the first to make an organic substance from an inorganic substance. This was the beginning of the end of the theory of vitalism: the idea that organic and inorganic materials differed essentially by the presence of the “vital force”– present only in organic material. To his mentor Berzelius:“I can make urea without thereby needing to have kidneys, or anyhow, an animal, be it human or dog" Friedrich Wöhler, 1880-1882 Polytechnic School in Berlin Support for vitalism remained until 1845, when Kolbe synthesized acetic acid Polytechnic School at Kassel from carbon disulfide University of Göttingen Fischer synthesis of glucose PhHN N O N O NHPh Br aq. HCl Δ PhNH2NH2 HO H HO H O Br "α−acrose" H OH H OH H OH H OH CH2OH CH2OH α−acrosazone α−acrosone an “osazone” (osazone test for reducing sugars) Zn/AcOH OH OH CO2H CHO O HO H HO H OH Br2 HO H HO H Na-Hg HO H HNO3 HO H H OH H OH H OH H OH then resolve H O+ H OH via strychnine H OH H OH 3 H OH CH2OH salts CH2OH CH OH CH2OH 2 D-Mannonic acid DL-Mannose DL-Mannitol DL-Fructose quinoline • Established stereochemical relationship CO2H CHO H OH H OH between mannose and glucose Na-Hg HO H HO H (part of Fischer proof) H OH + H OH H3O • work mechanisms from acrosazone on H OH H OH Emil Fischer, 1852-1919 CH2OH CH2OH University of Munich (1875-81) D-Gluconic acid D-Glucose University of Erlangen (1881-88) University of Würzburg (1888-92) University of Berlin (1892-1919) Fischer, E. -

(Punica Granatum L.) Peel Waste: a Review
molecules Review Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review Tandokazi Pamela Magangana 1,2 , Nokwanda Pearl Makunga 1 , Olaniyi Amos Fawole 3 and Umezuruike Linus Opara 2,* 1 Department of Botany and Zoology, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (T.P.M.); [email protected] (N.P.M.) 2 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Horticultural Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Stellenbosch 7602, South Africa 3 Postharvest Research Laboratory, Department of Botany and Plant Biotechnology, University of Johannesburg, P.O. Box 524, Auckland Park, Johannesburg 2006, South Africa; [email protected] * Correspondence: [email protected]; Tel.: +27-21-808-4064 or +27-21-808-3743 Academic Editors: Ana Barros and Irene Gouvinhas Received: 5 September 2020; Accepted: 7 October 2020; Published: 14 October 2020 Abstract: Pomegranate peel has substantial amounts of phenolic compounds, such as hydrolysable tannins (punicalin, punicalagin, ellagic acid, and gallic acid), flavonoids (anthocyanins and catechins), and nutrients, which are responsible for its biological activity. However, during processing, the level of peel compounds can be significantly altered depending on the peel processing technique used, for example, ranging from 38.6 to 50.3 mg/g for punicalagins. This review focuses on the influence of postharvest processing factors on the pharmacological, phytochemical, and nutritional properties of pomegranate (Punica granatum L.) peel. Various peel drying strategies (sun drying, microwave drying, vacuum drying, and oven drying) and different extraction protocols (solvent, super-critical fluid, ultrasound-assisted, microwave-assisted, and pressurized liquid extractions) that are used to recover phytochemical compounds of the pomegranate peel are described. -

Identification of L-Iduronic Acid As a Constituent of the Major Extracellular Polysaccharide Produced by Butyriuibrio Fibrisoluens Strain X6C61
FEMS Microbiology Letters 51 (1988) 1-6 Published by Elsevier FEM 03178 Identification of L-iduronic acid as a constituent of the major extracellular polysaccharide produced by Butyriuibrio fibrisoluens strain X6C61 Robert J. Stack, Ronald D. Plattner and Gregory L. Cote Northern Regional Research Cen:er,. Agricultural Research Service, u.s. Department ofAgriculture, 181:J N. University St., Peoria, 11., U.S.A. Received 8 February 1988 Accepted 12 February 1988 Key words: L-iduronic acid; Iduronolactone; Butyriuibrio fibrisolvens; Rumen; Extracellular polysaccharide 1. SUMMARY 2. INTRODUCTION Butyrivibrio fibrisolvens strain X6C61 produces Butyrivibrio fibrisolvens is one of the most fre two extracellular polysaccharides (EPS-I and EPS quently isolated species of ruminal bacteria [1,2]. II) separable by anion-exchange chromatography. There are, at present, a large number of isolates The neutral sugar constituents of EPS-I were iden that fit the species description, with correspond tified by gas-liquid chromatography (GLC) as the ingly wide range of reported metabolic activities alditol acetates of rhamnose, mannose, galactose, [3]. glucose, and an unidentified component. These Stack [4] has recently reported that many strains results were confirmed using thin-layer chro of B. fibrisolvens produce EPS containing unusual matography (TLC). Neutral sugar analysis of monosaccharide constituents. For example, B. EPS-II, which eluted from DEAE-Sephadex at 0.4 fibrisolvens strain CF3 produces an EPS which M NaCl, yielded the alditol acetates of rhamnose, contains L-altrose [5], the first reported occurrence galactose, glucose, and idose. However, idose was of this hexose in nature. However, analysis of not found when hydrolysates of EPS-II were L-altrose-containing EPS by conventional alditol analysed by TLC. -

Ii- Carbohydrates of Biological Importance
Carbohydrates of Biological Importance 9 II- CARBOHYDRATES OF BIOLOGICAL IMPORTANCE ILOs: By the end of the course, the student should be able to: 1. Define carbohydrates and list their classification. 2. Recognize the structure and functions of monosaccharides. 3. Identify the various chemical and physical properties that distinguish monosaccharides. 4. List the important monosaccharides and their derivatives and point out their importance. 5. List the important disaccharides, recognize their structure and mention their importance. 6. Define glycosides and mention biologically important examples. 7. State examples of homopolysaccharides and describe their structure and functions. 8. Classify glycosaminoglycans, mention their constituents and their biological importance. 9. Define proteoglycans and point out their functions. 10. Differentiate between glycoproteins and proteoglycans. CONTENTS: I. Chemical Nature of Carbohydrates II. Biomedical importance of Carbohydrates III. Monosaccharides - Classification - Forms of Isomerism of monosaccharides. - Importance of monosaccharides. - Monosaccharides derivatives. IV. Disaccharides - Reducing disaccharides. - Non- Reducing disaccharides V. Oligosaccarides. VI. Polysaccarides - Homopolysaccharides - Heteropolysaccharides - Carbohydrates of Biological Importance 10 CARBOHYDRATES OF BIOLOGICAL IMPORTANCE Chemical Nature of Carbohydrates Carbohydrates are polyhydroxyalcohols with an aldehyde or keto group. They are represented with general formulae Cn(H2O)n and hence called hydrates of carbons. -

Oxidation of Uronic Acids by a Large Excess of Glucose Oxidase Preparations
1 •k J. Appl. Glycosci., Vol.46, No.1, p.1-7 (1999)•l Oxidation of Uronic Acids by a Large Excess of Glucose Oxidase Preparations Mikihiko Kobayashi,* Hirofumi Nishihara1 and Shoichi Kobayashi National Food Research Institute (2-1-2, Kannondai, Tsukuba 305-8642, Japan)1 Department of Applied Bioresource Science, School of Agriculture, Ibaraki University (3998 Ami-machi, Ibaraki 300-0393, Japan) Three commercial enzyme preparations of glucose oxidase (GOD) showed an oxidizing ability of glucuronic acid and galacturonic acid to form sugar acids. The optimum conditions of GOD reaction were at pH 8.0 and 50•Ž, whereass those of uronic acid oxidase (UOD) reaction were at pH 3.5 and 40•Ž. In spite of extensive attempts to separate GOD from UOD, the isolation of each activity was unsuccessful, and UOD reaction resulted from the wide substrate specificity of GOD reaction. More than 6-fold higher Km values for glucuronic acid and galacturonic acid than for glucose might suggest that UOD reaction was the alternative reaction of GOD enzyme. However, a more definite conclusion on UOD activity should be drawn from further studies. The reaction products from uronic acids were analyzed by HPLC and paper chromatography, and some products were shown to be identical with sugar acids. Beet pulp contained a large amount of uronic oxidation of uronic acids with glucose oxidase acids, which were found not only in the pectic (GOD) preparation from commercial sources. fraction, but also in the hemicellulose and cellulose, fractions.1) Although the effective MATERIALS AND METHODS saccharification of beet pulp might increase biomass content about 1.4-fold for the ethanol Materials. -

Structure and Bioactivity of a Polysaccharide Containing Uronic Acid
Carbohydrate Polymers 152 (2016) 222–230 Contents lists available at ScienceDirect Carbohydrate Polymers j ournal homepage: www.elsevier.com/locate/carbpol Structure and bioactivity of a polysaccharide containing uronic acid from Polyporus umbellatus sclerotia a,b b,c,∗ c c,d b,∗ Pengfei He , Anqiang Zhang , Fuming Zhang , Robert J. Linhardt , Peilong Sun a College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou 310032, China b College of Ocean, Zhejiang University of Technology, Hangzhou 310032, China c Departments of Chemistry and Chemical Biology and Biomedical Engineering, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA d Department of Biological Science, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA a r t i c l e i n f o a b s t r a c t Article history: Polyporus umbellatus is a medicinal fungus, has been used in traditional Chinese medicine for thou- Received 22 April 2016 sands years for treatment of edema, scanty urine, vaginal discharge, jaundice and diarrhea. The structure Received in revised form 29 June 2016 of a soluble polysaccharide (named PUP80S1), purified from the sclerotia of Polyporus umbellatus was Accepted 3 July 2016 elucidated by gas chromatography (GC), GC–mass spectrometry and nuclear magnetic resonance spec- Available online 4 July 2016 troscopy. PUP80S1 is a branched polysaccharide containing approximately 8.5% uronic acid and having an average molecular weight of 8.8 kDa. Atomic force microscopy of PUP80S1 reveals a globular chain confor- Keywords: mation in water. Antioxidant tests, Oxygen radical absorption capacity and 2,2-diphenyl-1-picrylhydrazyl Polyporus umbellatus Polysaccharide radical scavenging assays indicate that PUP80S1 possesses significant antioxidant activity.