Prnpolicy Review & News
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

03.031 Socc04 Final 2(R)
STATEOF CENTER CITY 2008 Prepared by Center City District & Central Philadelphia Development Corporation May 2008 STATEOF CENTER CITY 2008 Center City District & Central Philadelphia Development Corporation 660 Chestnut Street Philadelphia PA, 19106 215.440.5500 www.CenterCityPhila.org TABLEOFCONTENTSCONTENTS INTRODUCTION 1 OFFICE MARKET 2 HEALTHCARE & EDUCATION 6 HOSPITALITY & TOURISM 10 ARTS & CULTURE 14 RETAIL MARKET 18 EMPLOYMENT 22 TRANSPORTATION & ACCESS 28 RESIDENTIAL MARKET 32 PARKS & RECREATION 36 CENTER CITY DISTRICT PERFORMANCE 38 CENTER CITY DEVELOPMENTS 44 ACKNOWLEDGEMENTS 48 Center City District & Central Philadelphia Development Corporation www.CenterCityPhila.org INTRODUCTION CENTER CITY PHILADELPHIA 2007 was a year of positive change in Center City. Even with the new Comcast Tower topping out at 975 feet, overall office occupancy still climbed to 89%, as the expansion of existing firms and several new arrivals downtown pushed Class A rents up 14%. For the first time in 15 years, Center City increased its share of regional office space. Healthcare and educational institutions continued to attract students, patients and research dollars to downtown, while elementary schools experienced strong demand from the growing number of families in Center City with children. The Pennsylvania Convention Center expansion commenced and plans advanced for new hotels, as occupancy and room rates steadily climbed. On Independence Mall, the National Museum of American Jewish History started construction, while the Barnes Foundation retained designers for a new home on the Benjamin Franklin Parkway. Housing prices remained strong, rents steadily climbed and rental vacancy rates dropped to 4.6%, as new residents continued to flock to Center City. While the average condo sold for $428,596, 115 units sold in 2007 for more than $1 million, double the number in 2006. -

Pennsylvania's Largest Employers (At Least 1,000 Employees)
Pennsylvania's Largest Employers (At Least 1,000 Employees) 1st Quarter, 2018 Combined Government Ownerships Center for Workforce Information & Analysis (877) 4WF-DATA • www.workstats.dli.pa.gov • [email protected] September 2018 Rank Employer Rank Employer 1 Federal Government 51 ACME Markets Inc 2 State Government 52 Aerotek Inc 3 Wal-Mart Associates Inc 53 Geisinger Medical Center 4 Trustees of the University of PA 54 Reading Hospital 5 City of Philadelphia 55 Dolgencorp LLC 6 Pennsylvania State University 56 Carnegie Mellon University 7 Giant Food Stores LLC 57 Abington Memorial Hospital 8 School District of Philadelphia 58 FedEx Ground Package System Inc 9 UPMC Presbyterian Shadyside 59 Highmark Inc 10 United Parcel Service Inc 60 Kohl's Department Stores Inc 11 PNC Bank NA 61 Rite Aid of Pennsylvania Inc 12 University of Pittsburgh 62 Marmaxx Operating Corporation 13 Lowe's Home Centers LLC 63 The Hershey Company 14 Weis Markets Inc 64 Wells Fargo NA 15 The Children's Hospital of Philadelphia 65 Temple University Hospital Inc 16 Comcast Cablevision Corp (PA) 66 York Hospital 17 Home Depot USA Inc 67 SmithKline Beecham Corporation 18 PA State System of Higher Education 68 Starbucks Corporation 19 Giant Eagle Inc 69 Boscov's Department Store LLC 20 Amazon.com DEDC LLC 70 School District of Pittsburgh 21 The Vanguard Group Inc 71 UPMC Pinnacle Hospitals 22 Target Corporation 72 Geisinger Clinic 23 Merck Sharp & Dohme Corporation 73 Dick's Sporting Goods Inc 24 Western Penn Allegheny Health 74 Hershey Entertainment & Resorts Co 25 -

Name and Title Company and Work Address Email Phone 2020
Executive Leadership Institute for Women 2020 Philadelphia Class List Name and Title Company and Work Address Email Phone Cigna Michele Adams 215-761-1467 1601 Chestnut Street, TL 14A [email protected] Senior Director, Accounting Policy 267-418-3629 (c) Philadelphia, PA 19192 KPMG, LLP Lauren Albertson 267-256-3183 1601 Market Street [email protected] Senior Manager 215-817-0889 (c) Philadelphia, PA 07677 KPMG, LLP Rupali Amin 267-256-3221 1601 Market Street [email protected] Managing Director 267-210-4331 (c) Philadelphia, PA 07677 KPMG, LLP Abigail (Abby) Aungst 30 North Third Street, Suite 1000 [email protected] 717-507-7707 (c) Audit Senior Manager Harrisburg, PA 17101 Aramark Kelly Banaszak 267-671-4469 1101 Market St [email protected] Director of Communications 609-760-3332 (c) Philadelphia, PA 19107 Exelon Corporation Anne Bancroft 10 S. Dearborn St [email protected] 610-812-5454 (c) Associate General Counsel Chicago, IL 60603 Aramark Jennifer Bloom 215-238-8143 1101 Market St [email protected] Finance Director 215-779-1025 (c) Philadelphia, PA 19107 Geisinger Hannah Bobrowski 570-271-5417 100 North Academy Ave, MC 28-10 [email protected] Associate Vice President, Achieving Excellence 570-926-3071 (c) Danville, PA 17822 Independence Blue Cross Roslyn Boskett 1900 Market St, 7th Floor [email protected] 856-986-9814 (c) Director, Contact Center Philadelphia, PA 19103 KPMG, LLP Kelli Brown 1601 Market Street [email protected] 610-256-0628 (c) Senior Manager Audit Philadelphia, PA 07677 -

For Academic Partners
In collaboration with our academic partners in colleges, schools, centers, and offices across the CREATING AN University, the Steinbright Career Development Center was able to produce exceptional results for Drexel students in 2017–2018. Our combined effort IMPACT TOGETHER to help students build their professional skills, explore potential career paths, and better prepare themselves for success after graduation continues to yield positive outcomes that illustrate the strength of a focused and united Drexel academic community. The Steinbright team continues to work with our academic partners to maximize the work experience and professional development our students receive from our cooperative education program when evaluating, revising, and constructing Drexel’s classroom curricula. By improving the alignment and cohesiveness between the knowledge students learn in class with the experiences they have on co-op, we are able to greatly expand the capacity for student learning. The continued success of our cooperative education program, along with the resulting positive effects upon University curricula, can in large part be attributed to the effectiveness of this coordination between Steinbright and our academic partners. We are committed to further developing this important relationship and leveraging our strengths to enhance student success at Drexel. CO-OP BY THE NUMBERS 2017–2018 HIGHLIGHTS Total students employed ......................................... 5,325 Students employed internationally .............................. 197 Student employment rate .......................................98.2% Percentage of co-op jobs that were paid positions .....82.4% Unique co-op employers ....................................... 1,566 Number of countries w/ co-ops ................................... 39 96% 95% 88% Number of states w/ co-ops ........................................ 31 Number of COOP 101 sections ................................. 141 of graduates of co-op employers of co-op students Number of COOP 101 students ............................. -

2016 Annual Report
NOTES FROM THE FIELD 28-Page Book for 2016 Girl Scouts of Eastern Pennsylvania THIS ANNUAL REPORT BOOK IS PROPERTY OF Girl Scouts of Eastern Pennsylvania PERTINENT COORDINATES Girl Scouts of Eastern Pennsylvania 330 Manor Road, Miquon, Pennsylvania 19444 40.070206, -75.252716 FOR INITIAL RECORDS GIRL SCOUTS OF EASTERN PENNSYLVANIA, INC. (“GSEP”) OPERATES AS AN INDEPENDENT, NONPROFIT ORGANIZATION CHARTERED BY THE NATIONAL GIRL SCOUTS OF THE USA TO PROVIDE LEADERSHIP DEVELOPMENT OPPORTUNITIES FOR GIRLS IN NINE COUNTIES: BERKS, BUCKS, CARBON, CHESTER, DELAWARE, LEHIGH, MONTGOMERY, NORTHAMPTON, AND PHILADELPHIA. THE GIRL SCOUT PROMISE ON MY HONOR, I WILL TRY: TO SERVE GOD AND MY COUNTRY, TO HELP PEOPLE AT ALL TIMES, AND TO LIVE BY THE GIRL SCOUT LAW. IN THE EVENT OF MISPLACEMENT IF FOUND PLEASE CONTACT @ IS HENCE, THERE ISN’T A HANDSOME REWARD WAITING. WWW.GSEP.ORG GIRL SCOUTS OUR MISSION: GIRL SCOUTING BUILDS GIRLS OF COURAGE, CONFIDENCE, AND CHARACTER WHO MAKE THE WORLD A BETTER PLACE. CONTENTS: 01. EXECUTIVE MESSAGE 06. TAKE THE LEAD 2016 02. MEMBERSHIP 07. THANK YOU TO OUR DONORS 03. LAND 08. BOARD OF DIRECTORS 04. CAMPAIGN FOR GIRLS 09. OUR FOOTPRINT 05. FINANCE THE GIRL SCOUT LAW: I WILL DO MY BEST TO BE HONEST AND FAIR, FRIENDLY AND HELPFUL, CONSIDERATE AND CARING, COURAGEOUS AND STRONG, AND RESPONSIBLE FOR WHAT I SAY AND DO, AND TO RESPECT MYSELF AND OTHERS, RESPECT AUTHORITY, USE RESOURCES WISELY, MAKE THE WORLD A BETTER PLACE, AND BE A SISTER TO EVERY GIRL SCOUT. GIRL SCOUTS OF EASTERN PENNSYLVANIA. PRINTED MAY 2017. EXECUTIVE MESSAGE This annual report is the final volume of a three-year series documenting Girl Scouts of Eastern Pennsylvania’s Outdoor Program Vision and our journey to position GSEP as the premier leadership experience for girls. -

PSPS Annual Sponsorship Packages
PSPS Annual Sponsorship Packages LEADERSHIP - $12,000 (Limit: 1) • Speaking opportunity - Introduction of Leadership Forum • Sponsor can host a virtual break out networking session 30 speaker (3-5 minutes) minutes prior to a mutually agreed upon Quarterly Program based on a first come/first serve basis • Opportunity to submit 2-minute long video commercial to be played during the Leadership Forum that promotes • Will receive registration list (name, title, company) for each your company/offering Quarterly Program, Summer Social, and Holiday Party one • 15 Virtual tickets to the Leadership Forum week prior to each event • Company logo included in all email communications • Full Page Ad and logo recognition in the Leadership Forum Program Booklet • Company logo on website and all virtual event signage/PPTs • Verbal recognition at Leadership Forum and Quarterly Programs • Opportunity to submit one article for a quarterly PSPS • 4 tickets to Summer Social and 4 tickets to Holiday Party Newsletter (Article must be submitted by deadline and approved by PSPS prior to publication) • Eight (8) tickets to each Quarterly Program • • Opportunity to submit 1-minute long video commercial to be Sponsor spotlight in PSPS Newsletter once/year played at Quarterly Programs that promotes their • Featured in two PSPS Sponsor Spotlights in emails to members company/offering (limited spots available for each event) • Sponsor highlighted during New Member Webinars • Recognition four times per year on the PSPS LinkedIn Page • Sponsor has opportunity 4 times per -
President's Message Inside 4Q2019
Inside 4Q2019 2.......Template.for.Disaster 8.......Past.Events 3.......ACC.News 15.....Sponsors.for.2019 4.......Choice.of.Law.and.Covenants.. 16.....New.and.Returning.Members Not.to.Compete 18.....Upcoming.ACCGP.Events 5.......Member.Spotlight 18.....Chapter.Leadership 6.......Aileen.Schwartz.Forges.Relationships,.. Inspires.Change 7.......Association.of.Corporate.. Counsel’sTop.10.30-Somethings FOCUS President’s Message Peter A. Prinsen I am advised by our Membership Chair, Speaking of 2020, I’m thrilled CLE Institute on December Mike Eckhardt from Wawa, that the Greater to report that we have had an 10th, Morgan Lewis’ sponsor- Philadelphia Chapter’s membership is now at overwhelming response to our ship of Pro Bono efforts with over 1,600 members. Not only is the number 2020 sponsorship opportunities. Philadelphia VIP on December of our Chapter members outstanding, but the Like last year, we have been 11th and culminating with level of your involvement and commitment to oversubscribed in virtually all sponsorship our Annual Holiday Party and Board the Chapter is extraordinary as well. This year levels. Despite being oversubscribed, we Installation on December 12th at the we will have put on over 80 programs ranging continue to work hard to find opportunities Racquet Club of Philadelphia. from our Meet your Counterparts Programs for our sponsors to “squeeze them in” so that I am truly humbled to have been your to our In-House Counsel Conference (where they can enjoy the benefit of sponsorship of President for 2019. For 2020, the Chapter over 600 of you attended and participated in this fine Chapter. -

Young People Take Their Rightful Places As Full and Contributing Members of a World Class Workforce…
Young people take their rightful places as full and contributing members of a world class workforce… Philadelphia Youth Network ANNUAL REPORT 2006 Contents Letter 1 Project U-Turn 3-5 WorkReady 7-9 Partnerships 11-14 Financial Report 15-17 Partners/Supporters 18-19 Acknowledgements 20 Board/Staff 21 Youth Profiles Shardell Martin 2 Lance Lopez 4 Latoyia Hall 6 Chris Davidson 8 Samantha Domenech 10 Kadeem Washington 12 Dear Colleagues and Friends: The title of this year’s annual report has particular meaning for all of us at the Philadelphia Youth Network. The phrase derives from PYN’s new vision statement, devel- oped as part of our recent strategic planning process, which reads: All of our city’s young people take their rightful places as full and contributing members of a world class work force for the region. Implicit in this statement is a belief that too many of the young people we serve have limited access to workplace experience and social networks that can lead to occupations that are in demand today in our regional economy. And that in order to take their rightful places in that economy, we need to equip these young people with the skills and habits of work that will enable them to compete successfully for those jobs. While we are not yet close to achieving this vision, 2006 saw clear movement in that direction: Project U-Turn, the City-wide campaign to address Philadelphia’s dropout crisis, was launched in October. As a result, the City became aware of the magnitude of the problem, and also came to understand what can be done about it. -
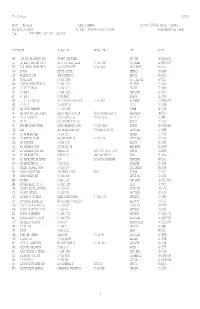
TPL Carrier Code Report
TPL-0021-M.txt 7/12/2021 Report : TPL-0021-M STATE OF ARKANSAS Run Date: 07/03/2021 Process : TPLJM002 MMIS REPORTING SYSTEM Run Time: 04:56:44 Location: TPL0021M CARRIER MASTER FILE - ALPHA Page: 1 REPORT PERIOD: 06/01/2021 - 06/30/2021 CARRIER NAME ADDRESS LINE 1 ADDRESS LINE 2 CITY ST ZIP HW6 1199 NATIONAL BENEFIT FUND 300 WEST 42ND STREET NEW YORK NY 10108-0933 UU 1ST HEALTH DEPT BMN HOSP O REF VF FOR OTHER CLAIMS P O BOX 10907 SCOTTSDALE AZ 85271-0907 EF5 21ST CENTURY HEALTH BENEFI 1760 MARKET STREET PO BOX 42930 PHILADELPHIA PA 19101 G96 55 PLUS BAPTIST HOSPITAL MEMMPHIS TN 38108 U24 65 SECURITY PLAN 3348 FONTAINE RD MEMPHIS TN 38116 U98 76 TRUCKSTOP P O BOX 15850 NO LITTLE ROCK AR 72231 S56 A AND H ADMINISTRATORS INC P O BOX 10223 MILWAUKEE WI 53223 Q19 A E STALEY MFG CO P O BOX 151 DECATUR IL 62525 UB A H AND L P O BOX 11111 FORT SCOTT KS 66701 RZ A H AND L PO BOX 88260 ATLANTA GA 30356 E85 A I G LIFE INSURANCE ACCIDENT AND HEALTH CLAIM PO BOX 15701 WILMINGTON DE 19850-5701 S82 A R G I S P O BOX 802516 DALLAS TX 75380 BJ7 AAA COOPER TRANSPORT P. O. BOX 6827 DOTHAN AL 36302 C8 AAA GROUP HOSPITAL INDEMNI AAA NEW MEXICO DIVISION 2201A SAN PEDRO BLVD NE ALBUQUERQUE NM 87110 U40 AAA OF WASHINGTON GROUP INSURANCE CO 222 NO MISSION WENATCHIE WA 98801 X95 AAC INC 16010 BARKERS PT LN 265 HOUSTON TX 77079 FI AARP HEALTHCARE OPTIONS UNITED HEALTHCARE CLAIMS P O BOX 740819 ATLANTA GA 30374-0819 CG8 ABAS AMER BENEFIT ADMIN SER 1733 PARK ST STE 300 NAPERVILLE IL 60564 J47 ABC ADVANTAGE PLAN P O BOX 2546 SHERMAN TX 75090 F99 ABC BENEFITS OF ARK 1500 RIVERFRONT DR P O BOX 3198 LITTLE ROCK AR 72203 S26 ABC INSURANCE P O BOX 3148 BALLWIN MO 63022 Q93 ABC INSURANCE TRUST 729 15TH ST. -

Prnpolicy Review & News
Policy Review & News Important information about Highmark Blue Shield April 2006 www.highmarkblueshield.comPRN In This Issue Blue Shield revises policy for collection of member deductibles, coinsurance, and copayments ......................................................1 MA Blue Shield seeks approval for UCR and PremierBlue Shield Look for this symbol for all reimbursement changes................................................................2 Medicare Advantage 1500A claim forms no longer available from Blue Shield ..............3 related information 2005 PRN index ..................................................insert Pages i-viii News Blue Shield revises policy for collection of member deductibles, coinsurance, and copayments In recent years, employer groups have been adding more cost-sharing components to their employees’ health care benefit plans. In turn, the frequency of collection of member deductibles, coinsurance, and copayments has increased for providers. To ensure that these member liabilities are collected appropriately, Highmark Blue Shield has revised its policy that outlines the procedures for collecting member deductibles, coinsurance, and copayments. This policy replaces the policy that was published in the June 2005 PRN (see “Deductible and coinsurance information available through various sources”). It permits facility, professional, and ancillary providers to collect the estimated member liability at the time of service. Highmark is a registered mark of Highmark Inc. Blue Shield and the Shield symbol are registered service marks of the Blue Cross and Blue Shield Association, an association of independent Blue Cross and Blue Shield Plans. PRN Here are the procedures: 1. Provider is permitted to collect deductibles, coinsurance, and copayments at the time of service as long as these criteria are met: a. Provider has as a standard operating procedure the policy to collect from all patients the amount due at the time of service and that all patients are notified in advance of the policy in writing. -

List of Top Pennsylvania Companies with Matching Gift Programs
List of top Pennsylvania Companies with Matching Gift Programs CORPORATION NAME CITY OF HEADQUARTERS Air Products and Chemicals, Inc. Allentown Lehigh Cement Company Allentown PPL Corporation Allentown Reliance Bank Altoona Sheetz, Inc. Altoona L.F. Driscoll Company Bala Cynwyd AMETEK, Inc. Berwyn Tyco Electronics Corporation Berwyn Just Born, Inc. Bethlehem Unisys Corporation Blue Bell Harsco Corporation Camp Hill Rite Aid Corporation Camp Hill Columbia Gas of Pennsylvania, Inc. Cannonsburg Giant Food Stores, Inc. Carlisle Ampco-Pittsburgh Corporation Carnegie Byers Choice Ltd. Chalfont Lebanon Mutual Insurance Company Cleona Quaker Chemical Corporation Conshohocken The Blommer Chocolate Company East Greenville Crayola LLC Easton Environmental Resources Management Limited Exton Applied Card Systems Glen Mills Henkel Corporation Gulph Mills The Hershey Company Hershey Eatn Park Hospitality Group Homestead ST Bancorp, Inc. Indiana Arkema Inc. King of Prussia Armstrong World Industries, Inc. Lancaster Auntie Annes, Inc. Lancaster Keystone Nazareth Bank Trust Lehigh Valley Teleflex Incorporated Limerick West Pharmaceutical Services, Inc. Lionville The Vanguard Group Malvern Parkvale Bank Monroeville Michael Baker Corporation Moon Township C.F. Martin Company, Inc. Nazareth SAP America, Inc. Newtown Square Seton Company Norristown List of top Pennsylvania Companies with Matching Gift Programs Comcast Holdings Corporation Philadelphia GlaxoSmithKline Holdings (America) Inc. Philadelphia Independence Blue Cross, LLC Philadelphia Pepper Hamilton LLP Philadelphia Philadelphia 76ers, L.P. Philadelphia Philadelphia Eagles Philadelphia Philadelphia Flyers, L.P. Philadelphia Radian Group Inc. Philadelphia Rohm and Haas Company Philadelphia Sovereign Bancorp, Inc. Philadelphia Sunoco, Inc. Philadelphia Tasty Baking Company Philadelphia The Phillies Philadelphia White Dog Cafe Philadelphia Alcoa Corporation Pittsburgh Allegheny Technologies Incorporated Pittsburgh American Eagle Outfitters, Inc. Pittsburgh Bayer Corporation Pittsburgh BEA Systems, Inc. -

US MBD: Short-Term Incentive Report
iMercer.com https://www.imercer.com/default.aspx?page=partlist&surveyid=7480 US MBD: Short-Term Incentive Report Participant List 1-800 CONTACTS, INC. KBR, INC. - UPSTREAM 7-ELEVEN, INC. KBR, INC. - UPSTREAM, GAS MONETIZATION AAA NORTHERN CALIFORNIA, NEVADA AND UTAH KBR, INC. - UPSTREAM, OIL & GAS AAF INTERNATIONAL KBR, INC. - VENTURES AB MAURI FOOD INC. KELLOGG COMPANY ABBOTT LABORATORIES KELLOGG COMPANY - AUSTIN QUALITY FOODS ABBOTT LABORATORIES - PHARMACEUTICAL PRODUCTS GROUP KELLOGG COMPANY - FROZEN FOODS ABINGTON MEMORIAL HOSPITAL KELLOGG COMPANY - KASHI ABRAXAS PETROLEUM CORPORATION KELLOGG COMPANY - KEEBLER DIVISION ABRAZO HEALTH CARE KELLOGG COMPANY - NORTH AMERICA ABRAZO HEALTH CARE - ARROWHEAD HOSPITAL KELSEY-SEYBOLD CLINIC ABRAZO HEALTH CARE - MARYVALE HOSPITAL KELSEY-SEYBOLD CLINIC - KELSEYCARE ABRAZO HEALTH CARE - PARADISE VALLEY HOSPITAL KEMPER, A UNITRIN BUSINESS ABRAZO HEALTH CARE - PHOENIX BAPTIST HOSPITAL KENDLE INTERNATIONAL ABRAZO HEALTH CARE - PHOENIX HEALTH PLAN KENTUCKY BAPTIST CONVENTION ABRAZO HEALTH CARE - WEST VALLEY HOSPITAL KENTUCKY LOTTERY CORPORATION ABS CAPITAL PARTNERS KERN HEALTH SYSTEMS ABT ASSOCIATES INC. KERRY, INC. US ACCIDENT FUND INSURANCE COMPANY OF AMERICA KEWAUNEE SCIENTIFIC CORPORATION ACCO BRANDS CORPORATION KEYCORP ACCO BRANDS CORPORATION - AMERICAS KEYSTONE FOODS, LLC ACCOR NORTH AMERICA, INC. KEYSTONE FOODS, LLC - EQUITY GROUP, ALABAMA ACE USA KEYSTONE FOODS, LLC - M & M RESTAURANT SUPPLY ACH FOOD COMPANIES KFORCE INC. ACUITY KIK CUSTOM PRODUCTS ADITYA BIRLA MINACS KIMBERLY-CLARK CORPORATION