Mapping the Geographical Distribution of Lymphatic Filariasis in Zambia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
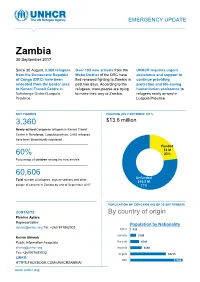
Zambia 30 September 2017
EMERGENCY UPDATE Zambia 30 September 2017 Since 30 August, 3,360 refugees Over 100 new arrivals from the UNHCR requires urgent from the Democratic Republic Moba District of the DRC have assistance and support to of Congo (DRC) have been fled renewed fighting to Zambia in continue providing relocated from the border area past two days. According to the protection and life-saving to Kenani Transit Centre in refugees, more people are trying humanitarian assistance to Nchelenge District/Luapula to make their way to Zambia. refugees newly arrived in Province. Luapula Province. KEY FIGURES FUNDING (AS 2 OCTOBER 2017) 3,360 $13.6 million requested for Zambia operation Newly-arrived Congolese refugees in Kenani Transit Centre in Nchelenge, Luapula province. 2,063 refugees have been biometrically registered Funded $3 M 60% 23% Percentage of children among the new arrivals Unfunded XX% 60,606 [Figure] M Unfunded Total number of refugees, asylum-seekers and other $10.5 M people of concern in Zambia by end of September 2017 77% POPULATION OF CONCERN (AS OF 30 SEPTEMBER) CONTACTS By country of origin Pierrine Aylara Representative Population by Nationality [email protected] Tel: +260 977862002 Other 415 Somalia 3199 Kelvin Shimoh Public Information Associate Burundi 4749 [email protected] Rwanda 6130 Tel: +260979585832 Angola 18715 LINKS: DRC 27398 HTTPS:FACEBOOK.COM/UNHCRZAMBIA/ www.unhcr.org 1 EMERGENCY UPDATE > Zambia / 30 September 2017 Emergency Response Luapula province, northern Zambia Since 30 August, over 3,000 asylum-seekers from the Democratic Republic of Congo (DRC) have crossed into northern Zambia. New arrivals are reportedly fleeing insecurity and clashes between Congolese security forces FARDC and a local militia groups in towns of Pweto, Manono, Mitwaba (Haut Katanga Province) as well as in Moba and Kalemie (Tanganyika Province). -

Fostering Accountability and Transparency (FACT) in Zambia Quarterly Report
Fostering Accountability and Transparency (FACT) in Zambia Quarterly Report January 1 to March 30, 2019 Youth Symposium Participants Outside FQM Trident Foundation Limited Offices after receiving training from one of FACT partners Submission Date: April 30, 2019 Submitted by: Chilufya Kasutu Agreement Number: Chief of Party AID-611-14-L-00001 Counterpart International, Zambia Email: [email protected] Submitted to: Edward DeMarco, USAID Zambia AOR This document was produced for review by the United States Agency for International Development, Zambia (USAID/Zambia). It was prepared by Counterpart International. ACRONYMS AND ABBREVIATIONS AOR Agreement Officer’s Representative ART Anti-Retroviral Treatment CCAs Community Conservation Areas CCPs Community Conservation Plans CFGs Community Forest Groups CEFTA Citizens Engagement in Fostering Transparency and Accountability COMACO Community Markets for Conservation CRB Community Resource Boards CSPR Civil Society for Poverty Reduction CSO Civil Society Organization DAC District Advocacy Committee DAMI District Alternative Mining Indaba DDCC District Development Coordinating Committee DEBS District Education Board Secretary DHO District Health Office DIM District Integrated Meetings EITI Extractive Industries Transparency Initiative ESSP Education and Skills Sector Plan FACT Fostering Accountability and Transparency FZS Frankfurt Zoological Society GPE Global Partnership for Education GRZ Government of the Republic of Zambia HCC Health Centre Committee HIV Human Immunodeficiency Virus LAG -

2.3.6 Zambia Border Crossing of Kazungula
2.3.6 Zambia Border Crossing of Kazungula Overview Daily Capacity Customs Clearance Other Relevant Information Overview Zambian Revenue Authority and Immigration staff are present at this crossing. It is a large office that is constantly congested with truck traffic. Access is good on tarmac roads from the north and south. From the North the nearest major center is Mongu. The eastern roads to Mongu are overall in good condition , even though the tarmac is damaged and has cracked in some places. Access from Livingstone is good and the road is in good condition. Pontoon operations are still operational though the World Bank funded bridge is now completed and awaits commissioning during the month of May 2021. Once the bridge has been commissioned, it is expected that traffic will increase at this border point as it is a shorter route from Livingstone City in Zambia to the City of Johannesburg in South Africa. Its about 900km from Kazungula border to Johannesburg via Botswana. Border Crossing Location and Contact Name of Border Crossing Zambia and Botswana: Kazungula Border Post Province or District Kazungula District, Southern Province Nearest Town or City with Distance from Border Crossing Livingstone (67 km) Latitude -17.78967 Longitude 25.26689 Managing Authority / Agency Zambian Revenue Authority (ZRA) Contact Person Mr. Emerson Chisuta, Station Master, (+260 978 582323) Travel Times Nearest International Airport Livingstone – Harry Mwaanga Nkumbula International Airport (73 km) Truck Travel Time: 3 hrs Car Travel time: 1.5hrs Nearest Port Durban, South Africa (1,825 km) Nearest location with functioning Livingstone (67 km) wholesale markets, or with significant manufacturing or Truck Travel Time: 3 hrs production capacity Car Travel time:1.5 hrs Other Information Roads are made of tarmac and in good condition. -

ZAMBIA HUMANITARIAN SITUATION REPORT 1 January to 30 June 2018
UNICEF ZAMBIA HUMANITARIAN SITUATION REPORT 1 January to 30 June 2018 Zambia Humanitarian Situation Report ©UNICEF Zambia/2017/Ayisi ©UNICEF REPORTING PERIOD: JANUARY - JUNE 2018 SITUATION IN NUMBERS Highlights 15,425 # of registered refugees in Nchelengue • As of 28 June 2018, a total of 15,425 refugees from the district Democratic Republic of the Congo (DRC) were registered at (UNHCR, Infographic 28 June 2018) Kenani transit centre in the Luapula Province of Zambia. • UNICEF and partners are supporting the Government of Zambia 79% to provide life-saving services for all the refugees in Kenani of registered refugees are women and transit centre and in the Mantapala permanent settlement area. children • More than half of the refugees have been relocated to Mantapala permanent settlement area. 25,000 • The set-up of basic services in Mantapala is drastically delayed # of expected new refugees from DRC in due to heavy rainfall that has made access roads impassable. Nchelengue District in 2018 • Discussions between UNICEF and the Government are under way to develop a transition and sustainability plan to ensure the US$ 8.8 million continuity of services in refugee hosting areas. UNICEF funding requirement UNICEF’s Response with Partners Funding Status 2018 UNICEF Sector Carry- forward Total Total amount: UNICEF Sector $0.2 m Funds received current Target Results* Target year: $2.5 m Results* Nutrition: # of children admitted for SAM 400 273 400 273 treatment Health: # of children vaccinated against 11,875 6,690 11,875 6,690 measles WASH: # of people provided with access to 15,000 9,253 25,000 15,425 Funding Gap: $6.1 m safe water =68% Child Protection: # of children receiving 5,500 3,657 9,000 4,668 psychosocial and/or other protection services Funds available include funding received for the current year as well as the carry-forward from the previous year. -

Visa Information for Zambia a Guide on the Requirements
UPDATED 24 JULY 2018 VISA INFORMATION FOR ZAMBIA A GUIDE ON THE REQUIREMENTS GENERAL INFORMATION ENTRY VISA – Please turn overleaf for a list of nationals requiring a visa prior to arrival and those exempt from visa requirements. Nationals not mentioned are eligible for a visa on arrival at our ports of entry. No entry visa required for SADC passports E-VISA APPLICATION – All persons who ordinarily require a visa to come to Zambia are eligible to apply for an e-Visa, LINK HERE ZAMBIAN MISSION ABROAD – Contact your local Zambia Diplomatic Mission to apply for a visa in person, list available HERE Check with your local embassy well before travelling or feel free to ask us and we will do our best to assist COST OF VISAS – Credit card facilities are not guaranteed at all points of entry - please carry sufficient US$’s to pay for visa’s if necessary ZAMBIA VISA COSTS (per passport - subject to change) SINGLE ENTRY $ 50 This visa is required should you wish to see the Falls from the Zimbabwe side or do a Chobe Day DOUBLE | MULTIPLE ENTRY – $ 80 Trip to Botswana – we suggest a multiple entry KAZA UNI-VISA – See further information below $ 50 KAZA UNI-VISA (40 nationalities are eligible) INFORMATION – Valid for 30 days & allows multiple cross-border visits between Zambia & Zimbabwe and one day-trip to Botswana. No overnight stays are allowed and multiple crossings to Botswana are not allowed on the same KAZA Uni-visa (not available online). AVAILABLE ON ARRIVAL – Livingstone International Airport, Victoria Falls International Airport, Harare -

Fifty Years of the Kasempa District, Zambia 1964 – 2014 Change and Continuity
FIFTY YEARS OF THE KASEMPA DISTRICT, ZAMBIA 1964 – 2014 CHANGE AND CONTINUITY. A case study of the ups and downs within a remote rural Zambian region during the fifty years since Independence. A descriptive analysis of its demography, geography, infrastructure, agricultural practice and present and traditional cultural aspects, including an account on the traditional ceremony of the installation of regional Headmen and the role and functions of the Kaonde clan structure. Dick Jaeger, 2015 [email protected] TABLE OF CONTENTS LIST OF MAPS AND FIGURES...........................................................................................................3 PART I 4 PREFACE – A WORD OF THANKS.....................................................................................................4 INTRODUCTION AND SUMMARY......................................................................................................6 CHAPTER 1. DEMOGRAPHIC CHANGES.......................................................................................10 ZAMBIA.............................................................................................................................10 KASEMPA DISTRICT........................................................................................................10 CHAPTER 2. AGRICULTURE............................................................................................................12 INTRODUCTION...............................................................................................................12 -

Ecological Changes in the Zambezi River Basin This Book Is a Product of the CODESRIA Comparative Research Network
Ecological Changes in the Zambezi River Basin This book is a product of the CODESRIA Comparative Research Network. Ecological Changes in the Zambezi River Basin Edited by Mzime Ndebele-Murisa Ismael Aaron Kimirei Chipo Plaxedes Mubaya Taurai Bere Council for the Development of Social Science Research in Africa DAKAR © CODESRIA 2020 Council for the Development of Social Science Research in Africa Avenue Cheikh Anta Diop, Angle Canal IV BP 3304 Dakar, 18524, Senegal Website: www.codesria.org ISBN: 978-2-86978-713-1 All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording or any information storage or retrieval system without prior permission from CODESRIA. Typesetting: CODESRIA Graphics and Cover Design: Masumbuko Semba Distributed in Africa by CODESRIA Distributed elsewhere by African Books Collective, Oxford, UK Website: www.africanbookscollective.com The Council for the Development of Social Science Research in Africa (CODESRIA) is an independent organisation whose principal objectives are to facilitate research, promote research-based publishing and create multiple forums for critical thinking and exchange of views among African researchers. All these are aimed at reducing the fragmentation of research in the continent through the creation of thematic research networks that cut across linguistic and regional boundaries. CODESRIA publishes Africa Development, the longest standing Africa based social science journal; Afrika Zamani, a journal of history; the African Sociological Review; Africa Review of Books and the Journal of Higher Education in Africa. The Council also co- publishes Identity, Culture and Politics: An Afro-Asian Dialogue; and the Afro-Arab Selections for Social Sciences. -

TECHNISCHE UNIVERSITÄT MÜNCHEN Role of Land Governance in Improving Tenure Security in Zambia: Towards a Strategic Framework F
TECHNISCHE UNIVERSITÄT MÜNCHEN Lehrstuhl für Bodenordnung und Landentwicklung Institut für Geodäsie, GIS und Landmanagement Role of Land Governance in Improving Tenure Security in Zambia: Towards a Strategic Framework for Preventing Land Conflicts Anthony Mushinge Vollständiger Abdruck der von der Ingenieurfakultät Bau Geo Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktor-Ingenieurs genehmigten Dissertation. Vorsitzender: Prof. Dr. Ir. Walter Timo de Vries Prüfer der Dissertation: 1. Prof. Dr.-Ing. Holger Magel 2. Prof. Dr. sc. agr. Michael Kirk (Philipps Universität Marburg) 3. Prof. Dr. Jaap Zevenbergen (University of Twente / Niederlande) Die Dissertation wurde am 25.04.2017 bei der Technischen Universität München eingereicht und durch die Ingenieurfakultät Bau Geo Umwelt am 25.08.2017 angenommen. Abstract Zambia is one of the countries in Africa with a high frequency of land conflicts. The conflicts over land lead to tenure insecurity. In response to the increasing number of land conflicts, the Zambian Government has undertaken measures to address land conflicts, but the measures are mainly curative in nature. But a conflict sensitive land governance framework should address both curative and preventive measures. In order to obtain insights about the actual realities on the ground, based on a case study approach, the research examined the role of existing state land governance framework in improving tenure security in Lusaka district, and established how land conflicts affect land tenure security. The research findings show that the present state land governance framework is malfunctional which cause land conflicts and therefore, tenure insecurity. The research further reveals that state land governance is characterised by defective legal and institutional framework and inappropriate technical (i.e. -

Report20 Uniting to End Malaria 501(C)3
PHOTO BY PAUL ISHII ANNUAL REPORT20 Uniting to End Malaria 501(c)3. EIN: 46-1380419 No one can foresee the duration or severity of COVID’s human and economic toll. But the malaria global health community agrees it will be disastrous to neglect or underinvest in malaria during this period, and thereby squander a decade of hard won progress. By some estimates, halting malaria intervention efforts could trigger a return to one million malaria deaths per year, a devastating mortality rate unseen since 2004. To that end many of our efforts last year were to strategically advocate for continued global malaria funding, as well as supporting COVID adjustments to ensure malaria projects were not delayed. Last year we supplied Personal Protection Equipment (PPE) to over 700 Rotary-funded community health workers (CHWs) in Uganda and Zambia; altered CHW The training to incorporate appropriate social distancing; conducted several webinars specifically focused on maintaining malaria financial support despite COVID; and we provided $50,000 to the Alliance for Malaria Prevention used for COVID/malaria public education in Africa. Jeff Pritchard Board Chair While our near-term work must accommodate pandemic restrictions, we are still firmly committed to our mission, “to generate a broad international Rotary campaign for the global elimination of malaria.” During the coming twelve months we intend to: • Implement a blueprint developed in 2020 for a large long-term Road malaria program with Rotary, the Bill & Melinda Gates Foundation, and World Vision, in the most underserved regions of Zambia’s Central and Muchinga Provinces, positively impacting nearly 1.4 million residents. -

Determinants of Spatio Temporal Variability of Water Quality in The
© University of Hamburg 2018 All rights reserved Klaus Hess Publishers Göttingen & Windhoek www.k-hess-verlag.de ISBN: 978-3-933117-95-3 (Germany), 978-99916-57-43-1 (Namibia) Language editing: Will Simonson (Cambridge), and Proofreading Pal Translation of abstracts to Portuguese: Ana Filipa Guerra Silva Gomes da Piedade Page desing & layout: Marit Arnold, Klaus A. Hess, Ria Henning-Lohmann Cover photographs: front: Thunderstorm approaching a village on the Angolan Central Plateau (Rasmus Revermann) back: Fire in the miombo woodlands, Zambia (David Parduhn) Cover Design: Ria Henning-Lohmann ISSN 1613-9801 Printed in Germany Suggestion for citations: Volume: Revermann, R., Krewenka, K.M., Schmiedel, U., Olwoch, J.M., Helmschrot, J. & Jürgens, N. (eds.) (2018) Climate change and adaptive land management in southern Africa – assessments, changes, challenges, and solutions. Biodiversity & Ecology, 6, Klaus Hess Publishers, Göttingen & Windhoek. Articles (example): Archer, E., Engelbrecht, F., Hänsler, A., Landman, W., Tadross, M. & Helmschrot, J. (2018) Seasonal prediction and regional climate projections for southern Africa. In: Climate change and adaptive land management in southern Africa – assessments, changes, challenges, and solutions (ed. by Revermann, R., Krewenka, K.M., Schmiedel, U., Olwoch, J.M., Helmschrot, J. & Jürgens, N.), pp. 14–21, Biodiversity & Ecology, 6, Klaus Hess Publishers, Göttingen & Windhoek. Corrections brought to our attention will be published at the following location: http://www.biodiversity-plants.de/biodivers_ecol/biodivers_ecol.php Biodiversity & Ecology Journal of the Division Biodiversity, Evolution and Ecology of Plants, Institute for Plant Science and Microbiology, University of Hamburg Volume 6: Climate change and adaptive land management in southern Africa Assessments, changes, challenges, and solutions Edited by Rasmus Revermann1, Kristin M. -

Profiles of Active Civil Society Organisations in North-Western, Copperbelt and Southern Provinces of Zambia
Profiles of Active Civil Society Organisations in North-Western, Copperbelt and Southern Provinces of Zambia On behalf of Implemented by Published by: Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH Registered offices Bonn and Eschborn, Germany Address Civil Society Participation Programme (CSPP) Mpile Office Park, 3rd floor 74 Independence Avenue Lusaka, Zambia P +260 211 250 894 E [email protected] I www.giz.de/en Programme: Civil society participation in governance reform and poverty reduction Author: Isaac Ngoma, GFA Consulting Group GmbH Editor: Markus Zwenke, GFA Consulting Group GmbH, Eulenkrugstraße 82, 22359 Hamburg, Germany Design/layout: GFA Consulting Group GmbH and IE Zhdanovich Photo credits/sources: GFA Consulting Group GmbH On behalf of German Federal Ministry for Economic Cooperation and Development (BMZ) As of June, 2021 TABLE OF CONTENT ACTIVE CIVIL SOCIETY ORGANISATIONS IN NORTH-WESTERN PROVINCE � � � � � �7 Dream Achievers Academy �������������������������������������������������������������������������������������������������������������������������� 8 Anti-voter Apathy Project ���������������������������������������������������������������������������������������������������������������������������� 9 Mentra Youth Zambia . 10 The Africa Youth Initiative Network �������������������������������������������������������������������������������������������������������� 11 Radio Kabangabanga ���������������������������������������������������������������������������������������������������������������������������������� -

The Case of Honey in Zambia the Case
Small-scale with outstanding economic potential enterprises woodland-based In some countries, honey and beeswax are so important the term ‘beekeeping’ appears in the titles of some government ministries. The significance of honey and beeswax in local livelihoods is nowhere more apparent than in the Miombo woodlands of southern Africa. Bee-keeping is a vital source of income for many poor and remote rural producers throughout the Miombo, often because it is highly suited to small scale farming. This detailed Non-Timber Forest Product study from Zambia examines beekeeping’s livelihood role from a range of perspectives, including market factors, production methods and measures for harnessing beekeeping to help reduce poverty. The caseThe in Zambia of honey ISBN 979-24-4673-7 Small-scale woodland-based enterprises with outstanding economic potential 9 789792 446739 The case of honey in Zambia G. Mickels-Kokwe G. Mickels-Kokwe Small-scale woodland-based enterprises with outstanding economic potential The case of honey in Zambia G. Mickels-Kokwe National Library of Indonesia Cataloging-in-Publication Data Mickels-Kokwe, G. Small-scale woodland-based enterprises with outstanding economic potential: the case of honey in Zambia/by G. Mickels-Kokwe. Bogor, Indonesia: Center for International Forestry Research (CIFOR), 2006. ISBN 979-24-4673-7 82p. CABI thesaurus: 1. small businesses 2. honey 3. beekeeping 4. commercial beekeeping 5. non- timber forest products 6. production 7. processing 8. trade 9.government policy 10. woodlands 11. case studies 12. Zambia I. Title © 2006 by CIFOR All rights reserved. Published in 2006 Printed by Subur Printing, Jakarta Design and Layout by Catur Wahyu and Eko Prianto Cover photo by Mercy Mwape of the Forestry Department of Zambia Published by Center for International Forestry Research Jl.