Arachnozoogeographical Analysis of the Boundary Between Eastern Palearctic and Indomalayan Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development Or Despoilation? - Krishnakumar
Andaman Islands: Development or Despoilation? - Krishnakumar DEVELOPMENT OR DESPOILATION? The Andaman Islands under colonial and postcolonial regimes M.V. KRISHNAKUMAR Jawaharlal Nehru University, New Delhi <[email protected]> Abstract The last quarter of the 19th Century marked an important watershed in the history of the Andaman Islands. The establishment of a penal settlement and an Imperial forestry service, along with other radical changes in the islands’ traditional economy and society, completely transformed the basic pattern of their forest resource use and entire system of forest management. These colonial policies, directly or indirectly, had a drastic impact on the indigenous population and island ecology. This article analyses the sources of environmental change in the Andaman Islands by examining the general ecological impacts of the state initiated development programmes. It also analyses the ‘civilising missions’ and forestry operations undertaken by British colonial administrators as well as the Indian state’s development initiatives under the ‘Five Year Plans’ that followed Indian independence in 1947. Keywords Andaman Islands, forestry, development, environmental change, Andaman tribes Introduction On December 26th 2004 a tsunami triggered by an earthquake off the south east coast of Sumatra swept across the Indian Ocean swamping many low-lying coastal areas and causing death, destruction of properties and infrastructure and despoliation of crops. Amongst those territories worst affected by the surge were the Andaman and Nicobar Islands. When Indian prime minister Dr Manmohan Singh visited the islands in the immediate aftermath of the flooding he identified that the project to reconstruct and rehabilitate coastal areas of islands provided the opportunity for a ‘New Andamans’ in which sustainable agriculture and fishery enterprises could exist in harmony with the natural environment. -

The Andaman Islands Penal Colony: Race, Class, Criminality, and the British Empire*
IRSH 63 (2018), Special Issue, pp. 25–43 doi:10.1017/S0020859018000202 © 2018 Internationaal Instituut voor Sociale Geschiedenis. This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http:// creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited. The Andaman Islands Penal Colony: Race, Class, Criminality, and the British Empire* C LARE A NDERSON School of History, Politics and International Relations University of Leicester University Road, Leicester LE1 7RH, UK E-mail: [email protected] ABSTRACT: This article explores the British Empire’s configuration of imprisonment and transportation in the Andaman Islands penal colony. It shows that British governance in the Islands produced new modes of carcerality and coerced migration in which the relocation of convicts, prisoners, and criminal tribes underpinned imperial attempts at political dominance and economic development. The article focuses on the penal transportation of Eurasian convicts, the employment of free Eurasians and Anglo-Indians as convict overseers and administrators, the migration of “volunteer” Indian prisoners from the mainland, the free settlement of Anglo-Indians, and the forced resettlement of the Bhantu “criminal tribe”.It examines the issue from the periphery of British India, thus showing that class, race, and criminality combined to produce penal and social outcomes that were different from those of the imperial mainland. These were related to ideologies of imperial governmentality, including social discipline and penal practice, and the exigencies of political economy. INTRODUCTION Between 1858 and 1939, the British government of India transported around 83,000 Indian and Burmese convicts to the penal colony of the Andamans, an island archipelago situated in the Bay of Bengal (Figure 1). -

Comparative Functional Morphology of Attachment Devices in Arachnida
Comparative functional morphology of attachment devices in Arachnida Vergleichende Funktionsmorphologie der Haftstrukturen bei Spinnentieren (Arthropoda: Arachnida) DISSERTATION zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) an der Mathematisch-Naturwissenschaftlichen Fakultät der Christian-Albrechts-Universität zu Kiel vorgelegt von Jonas Otto Wolff geboren am 20. September 1986 in Bergen auf Rügen Kiel, den 2. Juni 2015 Erster Gutachter: Prof. Stanislav N. Gorb _ Zweiter Gutachter: Dr. Dirk Brandis _ Tag der mündlichen Prüfung: 17. Juli 2015 _ Zum Druck genehmigt: 17. Juli 2015 _ gez. Prof. Dr. Wolfgang J. Duschl, Dekan Acknowledgements I owe Prof. Stanislav Gorb a great debt of gratitude. He taught me all skills to get a researcher and gave me all freedom to follow my ideas. I am very thankful for the opportunity to work in an active, fruitful and friendly research environment, with an interdisciplinary team and excellent laboratory equipment. I like to express my gratitude to Esther Appel, Joachim Oesert and Dr. Jan Michels for their kind and enthusiastic support on microscopy techniques. I thank Dr. Thomas Kleinteich and Dr. Jana Willkommen for their guidance on the µCt. For the fruitful discussions and numerous information on physical questions I like to thank Dr. Lars Heepe. I thank Dr. Clemens Schaber for his collaboration and great ideas on how to measure the adhesive forces of the tiny glue droplets of harvestmen. I thank Angela Veenendaal and Bettina Sattler for their kind help on administration issues. Especially I thank my students Ingo Grawe, Fabienne Frost, Marina Wirth and André Karstedt for their commitment and input of ideas. -

Russia's Boreal Forests
Forest Area Key Facts & Carbon Emissions Russia’s Boreal Forests from Deforestation Forest location and brief description Russia is home to more than one-fifth of the world’s forest areas (approximately 763.5 million hectares). The Russian landscape is highly diverse, including polar deserts, arctic and sub-arctic tundra, boreal and semi-tundra larch forests, boreal and temperate coniferous forests, temperate broadleaf and mixed forests, forest-steppe and steppe (temperate grasslands, savannahs, and shrub-lands), semi-deserts and deserts. Russian boreal forests (known in Russia as the taiga) represent the largest forested region on Earth (approximately 12 million km2), larger than the Amazon. These forests have relatively few tree species, and are composed mainly of birch, pine, spruce, fir, with some deciduous species. Mixed in among the forests are bogs, fens, marshes, shallow lakes, rivers and wetlands, which hold vast amounts of water. They contain more than 55 per cent of the world’s conifers, and 11 per cent of the world’s biomass. Unique qualities of forest area Russia’s boreal region includes several important Global 200 ecoregions - a science-based global ranking of the Earth’s most biologically outstanding habitats. Among these is the Eastern-Siberian Taiga, which contains the largest expanse of untouched boreal forest in the world. Russia’s largest populations of brown bear, moose, wolf, red fox, reindeer, and wolverine can be found in this region. Bird species include: the Golden eagle, Black- billed capercaillie, Siberian Spruce grouse, Siberian accentor, Great gray owl, and Naumann’s thrush. Russia’s forests are also home to the Siberian tiger and Far Eastern leopard. -

Estec Tecnologia
Ann. Natal Mus. Vol. 33(2) Pages 271-336 Pietermaritzburg October, 1992 An annotated check-list of Afrotropical harvestmen, excluding the Phalangiidae (Opiliones) by Wojciech Staregal (Natal Museum, P. B. 9070, 3200 Pietermaritzburg, South Africa) ABSTRACT A check-list of all known Afrotropical harvestmen, with exception of the family Phalangiidae, is provided. The following taxonomic changes are made: 28 generic and 45 specific synonymies, 37 new combinations and 3 new names. Generic synonymies: Cryptobunus Lawrence, 1931 = Arnatola Lawrence, 1931; Flavonuncia Lawrence, 1959 = Hovanuncia Lawrence, 1959; Spinirnontia Roewer, 1914, Tanalaius Roewer, 1914 & Triacurnontia Roewer, 1914 = Acurnontia Loman, 1898 (all Triaenonychidae); Arnbolotsca Roewer, 1949 = Erecanana Strand, 1911 (Erecananidae); Argobba Roewer, 1935 & Harsadia Roewer, 1935 = Arnhara Pavesi, 1897; Assiniana Roewer, 1914 & Aburitius Roewer, 1935 = Sassandria Roewer, 1912; Coelobunus Loman, 1902 = Dicoryphus Loman, 1902; Faradjea Roewer, 1950 = Ereala Roewer, 1950; Gobabisia Roewer, 1940 = Narnutonia Lawrence, 1931; Monorhabdiurn Loman, 1902, Metachilon Roewer, 1923, Parachilon Roewer, 1923, Bindercola Roewer, 1935, Tsadsea Roewer, 1935, Acanfhocoryphus Roewer, 1953, Sangalkarnia Roewer, 1953, Villersiella Roewer, 1953 & Kobacoryphus Roewer, 1961 all = Chilon Sorensen, 1896; Othrnar Roewer, 1935 = Orsirnonia Roewer, 1935; Phezilbus Roewer, 1935 = Sidarna Pavesi, 1895; Pseudoacaca Caporiacco, 1949 = Acaca Roewer, 1935; Pungoiella Roewer, 1914 & Pygoselenca Roewer, 1953 = Pungoica -
R Graphics Output
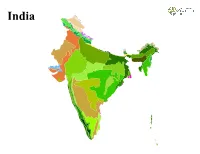
India India LEGEND Ecoregion Andaman Islands rain forests Mizoram−Manipur−Kachin rain forests Aravalli west thorn scrub forests Narmada Valley dry deciduous forests Baluchistan xeric woodlands Nicobar Islands rain forests Brahmaputra Valley semi−evergreen forests North Deccan dry deciduous forests Central Deccan Plateau dry deciduous forests North Western Ghats moist deciduous forests Central Tibetan Plateau alpine steppe North Western Ghats montane rain forests Chhota−Nagpur dry deciduous forests Northeast Himalayan subalpine conifer forests Chin Hills−Arakan Yoma montane forests Northeast India−Myanmar pine forests Deccan thorn scrub forests Northern Triangle temperate forests East Deccan dry−evergreen forests Northwestern Himalayan alpine shrub and meadows East Deccan moist deciduous forests Orissa semi−evergreen forests Eastern Himalayan alpine shrub and meadows Rann of Kutch seasonal salt marsh Eastern Himalayan broadleaf forests Rock and Ice Eastern Himalayan subalpine conifer forests South Deccan Plateau dry deciduous forests Godavari−Krishna mangroves South Western Ghats moist deciduous forests Himalayan subtropical broadleaf forests South Western Ghats montane rain forests Himalayan subtropical pine forests Sundarbans freshwater swamp forests Indus River Delta−Arabian Sea mangroves Sundarbans mangroves Karakoram−West Tibetan Plateau alpine steppe Terai−Duar savanna and grasslands Khathiar−Gir dry deciduous forests Thar desert Lower Gangetic Plains moist deciduous forests Upper Gangetic Plains moist deciduous forests Malabar Coast moist forests Western Himalayan alpine shrub and meadows Maldives−Lakshadweep−Chagos Archipelago tropical moist forests Western Himalayan broadleaf forests Meghalaya subtropical forests Western Himalayan subalpine conifer forests. -

North Yukon Planning Region Biophysical Landscape Classification
North Yukon Planning Region Biophysical Landscape Classification (Landscape Types): Overview, Methods and Reference Images T F DRA Updated October 20, 2005 Project Team • John Meikle, Yukon Environment • Marcus Waterreus, Yukon Environment • Shawn Francis, NYPC • Jeff Hamm, YLUPC • Nancy Steffen, GLL • Biophysical working group – initial terrain and bioclimate concepts STUDY AREA • North Yukon Planning Region (55,000 km2) • Taiga Cordillera Ecozone • 6 Ecoregions: Eagle Plains, Old Crow Flats, Old Crow Basin, North Ogilvie Mountains, Davidson Mountains, Richardson Mountains • Basin, Plateau and Mountain Landscapes STUDY AREA Physiographic Basin Units and Ecodistricts Mountain Basin Plateau Old Crow Plateau Mountain Mountain MAPPING CONCEPTS • Mountain landscapes organized by elevation (Bioclimate Zone); Plateau landscapes organized by relative moisture gradient (Ecosite) • Beyond major Basin landscapes, surficial materials not controlling factor (unglaciated) • Mapping has regional applications (1:100K- 1:250K) • Available data sources are major determinant of potential methods DATA INPUTS • EOSD (25m LANDSAT Extent of 250K Regional Terrain supervised classification) Map (raster) • 90m DEM (raster) • 250K regional terrain map North Yukon Planning Region (vector) Peel Watershed …..due to importance of Planning Region EOSD and DEM, raster mapping approach was chosen METHODS • Field reconnaissance; develop concepts • Create regional terrain and Bioclimate Zone map through manual interpretation (refine Ecoregions and Ecodistricts to 250K) -

New Distribution Records of the Intertidal Pseudoscorpion Parahya Submersa (Pseudoscorpiones: Parahyidae)
DOI: 10.18195/issn.0312-3162.23(4).2007.393-395 Records 01 the Western Australian A1useum 23: 393-395 New distribution records of the intertidal pseudoscorpion Parahya submersa (Pseudoscorpiones: Parahyidae) 1 2 3 Mark S. Harveyl, Julianne Waldock , Roy J. Teale and Jenni Webber I Department of Terrestrial Invertebrates, Western Australian Museum, Locked Bag 49, Welsh pool DC, Western Australia 6986, Australia 2 Biota Environmental Sciences I'ty Ud, 1'0 Box 155, Leederville, Western Australia 6903, Australia 11'.0. Box 2280, Humpty Doo, Northern Territory 0836, Australia Abstract - The intertidal pseudoscorpion Parahya submersa (Bristowe) is recorded from northern Australia for the first time, and new specimens are recorded from Singapore and New Caledonia. INTRODUCTION Family Parahyidae Harvey Intertidal pseudoscorpions are found in many Genus Parahya Beier parts of the world and some genera are totally or mostly restricted to seashore habitats. Prominent Parahya submersa (Bristowe) genera occurring in intertidal habitats are Garypus Figures 1, 2 L. Koch (Garypidae) (e.g. Lee, 1979), Halobisium Chamberlin (Neobisiidae) (e.g. Schulte, 1976) and Obisium submersum Bristowe, 1931: 465, figs 3-6; Paraliochthonius Beier (Chthoniidae) (e.g. Vachon, Roth and Brown, 1976: 128. 1960; Muchmore, 1967, 1972; Muchmore, 1984; Parahya pacifica Beier, 1957: 15-16, figs 4b, 5 Mahnert, 1986). Other less widespread genera such (synonymised by Harvey, 1991b: 288). as Nannochelifer Beier (Cheliferidae) and Parahya Beier (Parahyidae) also appear to be restricted to Parallya submersa (Bristowe): Harvey, 1991a: 315; the intertidal zone. The mechanisms by which these Harvey, 1991b: 288-290, figs 1-12; Harvey, 1992: small arachnids are able to survive in such figs 117-125; Judson, 1997: 42. -

Distribution of Cave-Dwelling Pseudoscorpions (Arachnida) in Brazil
2019. Journal of Arachnology 47:110–123 Distribution of cave-dwelling pseudoscorpions (Arachnida) in Brazil Diego Monteiro Von Schimonsky1,2 and Maria Elina Bichuette1: 1Laborato´rio de Estudos Subterraˆneos – Departamento de Ecologia e Biologia Evolutiva – Universidade Federal de Sa˜o Carlos, Rodovia Washington Lu´ıs, km 235, Caixa Postal 676, CEP 13565-905, Sa˜o Carlos, Sa˜o Paulo, Brazil; 2Programa de Po´s-Graduac¸a˜o em Biologia Comparada, Departamento de Biologia, Faculdade de Filosofia, Cieˆncias e Letras de Ribeira˜o Preto – Universidade de Sa˜o Paulo, Av. Bandeirantes, 3900, CEP 14040-901, Bairro Monte Alegre, Ribeira˜o Preto, Sa˜o Paulo, Brazil. E-mail: [email protected] Abstract. Pseudoscorpions are among the most diverse of the smaller arachnid orders, but there is relatively little information about the distribution of these tiny animals, especially in Neotropical caves. Here, we map the distribution of the pseudoscorpions in Brazilian caves and record 12 families and 22 genera based on collections analyzed over several years, totaling 239 caves from 13 states in Brazil. Among them, two families (Atemnidae and Geogarypidae) with three genera (Brazilatemnus Muchmore, 1975, Paratemnoides Harvey, 1991 and Geogarypus Chamberlin, 1930) are recorded for the first time in cave habitats as, well as seven other genera previously unknown for Brazilian caves (Olpiolum Beier, 1931, Pachyolpium Beier 1931, Tyrannochthonius Chamberlin, 1929, Lagynochthonius Beier, 1951, Neocheiridium Beier 1932, Ideoblothrus Balzan, 1892 and Heterolophus To¨mo¨sva´ry, 1884). These genera are from families already recorded in this habitat, which have their distributional ranges expanded for all other previously recorded genera. Additionally, we summarize records of Pseudoscorpiones based on previously published literature and our data for 314 caves. -

A Karyotype Study on the Pseudoscorpion Families Geogarypidae, Garypinidae and Olpiidae (Arachnida: Pseudoscorpiones)
Eur. J. Entomol. 103: 277–289, 2006 ISSN 1210-5759 A karyotype study on the pseudoscorpion families Geogarypidae, Garypinidae and Olpiidae (Arachnida: Pseudoscorpiones) 1,2 2 3 4 FRANTIŠEK ŠġÁHLAVSKÝ , JIěÍ KRÁL , MARK S. HARVEY and CHARLES R. HADDAD 1Department of Zoology, Faculty of Sciences, Charles University, Viniþná 7, CZ-128 44 Prague 2, Czech Republic; e-mail: [email protected] 2Laboratory of Arachnid Cytogenetics, Department of Genetics and Microbiology, Faculty of Sciences, Charles University, Viniþná 5, CZ-128 44 Prague 2, Czech Republic; e-mail: [email protected] 3Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australia 6986, Australia; e-mail: [email protected] 4Department of Zoology and Entomology, University of the Free State, P.O. Box 339, Bloemfontein 9300, South Africa; e-mail: [email protected] Key words. Pseudoscorpiones, Geogarypidae, Garypinidae, Olpiidae, karyotype, sex chromosomes, meiosis, chiasma frequency Abstract. The karyotypes of pseudoscorpions of three families, Geogarypidae, Garypinidae and Olpiidae (Arachnida: Pseudoscorpi- ones), were studied for the first time. Three species of the genus Geogarypus from the family Geogarypidae and 10 species belonging to 8 genera from the family Olpiidae were studied. In the genus Geogarypus the diploid chromosome numbers of males range from 15 to 23. In the family Olpiidae the male chromosome numbers vary greatly, from 7 to 23. The male karyotype of single studied member of the family Garypinidae, Garypinus dimidiatus, is composed of 33 chromosomes. It is proposed that the karyotype evolution of the families Geogarypidae and Olpiidae was characterised by a substantial decrease of chromosome numbers. -

Exploring the Evolution and Terrestrialization of Scorpions (Arachnida: Scorpiones) with Rocks and Clocks
Howard, R., Edgecombe, G. D., Legg, D., Pisani, D., & Lozano- Fernandez, J. (2019). Exploring the evolution and terrestrialization of scorpions (Arachnida: Scorpiones) with rocks and clocks. Organisms Diversity and Evolution, 19(1), 71-86. https://doi.org/10.1007/s13127- 019-00390-7 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1007/s13127-019-00390-7 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Springer at https://doi.org/10.1007/s13127-019-00390-7 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Organisms Diversity & Evolution (2019) 19:71–86 https://doi.org/10.1007/s13127-019-00390-7 REVIEW Exploring the evolution and terrestrialization of scorpions (Arachnida: Scorpiones) with rocks and clocks Richard J. Howard1,2,3 & Gregory D. Edgecombe2 & David A. Legg4 & Davide Pisani3 & Jesus Lozano-Fernandez5,3 Received: 3 August 2018 /Accepted: 2 January 2019 /Published online: 6 February 2019 # The Author(s) 2019 Abstract Scorpions (Arachnida: Scorpiones Koch, 1837) are an ancient chelicerate arthropod lineage characterised by distinctive subdi- vision of the opisthosoma and venomous toxicity. The crown group is represented by over 2400 extant species, and unambiguous fossil representatives are known at least from the Cretaceous Period. -

SA Spider Checklist
REVIEW ZOOS' PRINT JOURNAL 22(2): 2551-2597 CHECKLIST OF SPIDERS (ARACHNIDA: ARANEAE) OF SOUTH ASIA INCLUDING THE 2006 UPDATE OF INDIAN SPIDER CHECKLIST Manju Siliwal 1 and Sanjay Molur 2,3 1,2 Wildlife Information & Liaison Development (WILD) Society, 3 Zoo Outreach Organisation (ZOO) 29-1, Bharathi Colony, Peelamedu, Coimbatore, Tamil Nadu 641004, India Email: 1 [email protected]; 3 [email protected] ABSTRACT Thesaurus, (Vol. 1) in 1734 (Smith, 2001). Most of the spiders After one year since publication of the Indian Checklist, this is described during the British period from South Asia were by an attempt to provide a comprehensive checklist of spiders of foreigners based on the specimens deposited in different South Asia with eight countries - Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan and Sri Lanka. The European Museums. Indian checklist is also updated for 2006. The South Asian While the Indian checklist (Siliwal et al., 2005) is more spider list is also compiled following The World Spider Catalog accurate, the South Asian spider checklist is not critically by Platnick and other peer-reviewed publications since the last scrutinized due to lack of complete literature, but it gives an update. In total, 2299 species of spiders in 67 families have overview of species found in various South Asian countries, been reported from South Asia. There are 39 species included in this regions checklist that are not listed in the World Catalog gives the endemism of species and forms a basis for careful of Spiders. Taxonomic verification is recommended for 51 species. and participatory work by arachnologists in the region.