Vol 64:1761-1773
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dr Sanjaya Rajaram's Profile
Dr Sanjaya Rajaram’s profile Sanjaya Rajaram, born in India and a citizen of Mexico, graduated with his B.S. in Agriculture at the University of Gorakhpur and Masters in genetics and plant breeding at the Indian Agricultural Research Institute in 1962 and 1964 respectively. In 1965 he began his PhD in plant breeding at the University of Sydney on a scholarship from the Rotary Club of Narrabri under Dr. I.A. Watson who had been a fellow graduate student with Norman Borlaug at the University of Minnesota. Watson recommended him to Dr. Borlaug and Dr. Glenn Anderson at the International Maize and Wheat Improvement Center (CIMMYT) in Mexico, an action that set in motion Rajaram’s distinguished scientific career in wheat research. He implemented a major expansion of Borlaug’s ingenious shuttle-breeding approach in countries beyond Mexico. Dr Rajaram significantly advanced his mentor’s work in improving wheat varieties during a period that has been described as the “golden years” of wheat breeding and production. Like Borlaug, Rajaram had the extraordinary ability to visually identify and select for cross breeding the plant varieties possessing a range of desired characteristics, an ability that was essential to wheat breeding in the 1980s and ‘90s. Rajaram also launched efforts to expand the global scientific wheat network which had not been done before. This led to the accelerated development and worldwide spread of high- yielding wheat varieties, which has kept the expansion of global wheat production ahead of population growth and made wheat even more accessible to the world’s poor. He also realized the importance of nutrition to the poor and strongly supported research on micronutrient-enriched wheat varieties. -

Current Affairs
JUNE 2014 CURRENT AFFAIRS UPLOADED TO https://www.facebook.com/groups/BANKPOANDCLERK LIKE OUR FACEBOOK PAGE https://www.facebook.com/UPSCSSCBANKEXAMS EMAIL [email protected] [email protected] DOWNLOAD ALL THESE BOOKS FROM THE LINK GIVEN BELOW LAST 6 MONTH CURRENT AFFAIRS DECEMBER 1,2013 TO MAY 31,2014 SIZE-1.47MB PAGES-144 https://www.facebook.com/groups/BANKPOANDCLERK/680100562058494/ APRIL 2014 CURRENT AFFAIRS SIZE-623KB PAGES-32 https://drive.google.com/file/d/0BzU88b0FSZoNS1FUS0tWZms3dGM/edit?usp=sharing MARCH 2014 CURRENT AFFAIRS https://www.facebook.com/groups/BANKPOANDCLERK/645066935561857/ INDIAN POLITY BOOK USEFUL FOR SSC UPSC & OTHER GOVERNMENT EXAMS http://sh.st/ekp4w FEBRUARY 2014 CURRENT AFFAIRS https://www.facebook.com/groups/bankpoandclerk/630857426982808/ JANUARY 2014 CURRENT AFFAIRS https://www.facebook.com/groups/BANKPOANDCLERK/614450315290186/ OXFORD PRACTICE GRAMMAR BY John Eastwood PAGES-436 SIZE-4.79MB https://www.facebook.com/groups/BANKPOANDCLERK/596752047060013/ English Grammar in Use By Raymond Murphy A self-study reference and practice book https://www.facebook.com/groups/BANKPOANDCLERK/504274782974407/ OXFORD GUIDE TO ENGLISH GRAMMAR BY John Eastwood https://www.facebook.com/groups/BANKPOANDCLERK/516255345109684/ GEOMETRY BOOK FOR SSC AND UPSC EXAMS PAGES 646 SIZE - 6.49 MB https://www.facebook.com/groups/BANKPOANDCLERK/511616678906884/ IDIOMS AND PHRASES By DAVID HOLMES Pages 525 size - 1.63mb https://www.facebook.com/groups/BANKPOANDCLERK/524778944257324/ History of India and Indian National -

Advances in Wheat Genetics: from Genome to Field Proceedings of the 12Th International Wheat Genetics Symposium Advances in Wheat Genetics: from Genome to Field
Yasunari Ogihara · Shigeo Takumi Hirokazu Handa Editors Advances in Wheat Genetics: From Genome to Field Proceedings of the 12th International Wheat Genetics Symposium Advances in Wheat Genetics: From Genome to Field Yasunari Ogihara • Shigeo Takumi Hirokazu Handa Editors Advances in Wheat Genetics: From Genome to Field Proceedings of the 12th International Wheat Genetics Symposium Editors Yasunari Ogihara Shigeo Takumi Kihara Institute for Biological Research Graduate School of Agricultural Sciences Yokohama City University Kobe University Yokohama , Kanagawa , Japan Kobe , Hyogo , Japan Hirokazu Handa Plant Genome Research Unit National Institute of Agrobiological Sciences Tsukuba , Ibaraki , Japan ISBN 978-4-431-55674-9 ISBN 978-4-431-55675-6 (eBook) DOI 10.1007/978-4-431-55675-6 Library of Congress Control Number: 2015949398 Springer Tokyo Heidelberg New York Dordrecht London © The Editor(s) (if applicable) and the Author(s) 2015 . The book is published with open access at SpringerLink.com. Open Access This book is distributed under the terms of the Creative Commons Attribution Non- commercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited. All commercial rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specifi c statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Dear Aspirant with Regard
DEAR ASPIRANT HERE WE ARE PRESENTING YOU A GENRAL AWERNESS MEGA CAPSULE FOR IBPS PO, SBI ASSOT PO , IBPS ASST AND OTHER FORTHCOMING EXAMS WE HAVE UNDERTAKEN ALL THE POSSIBLE CARE TO MAKE IT ERROR FREE SPECIAL THANKS TO THOSE WHO HAS PUT THEIR TIME TO MAKE THIS HAPPEN A IN ON LIMITED RESOURCE 1. NILOFAR 2. SWETA KHARE 3. ANKITA 4. PALLAVI BONIA 5. AMAR DAS 6. SARATH ANNAMETI 7. MAYANK BANSAL WITH REGARD PANKAJ KUMAR ( Glory At Anycost ) WE WISH YOU A BEST OF LUCK CONTENTS 1 CURRENT RATES 1 2 IMPORTANT DAYS 3 CUPS & TROPHIES 4 4 LIST OF WORLD COUNTRIES & THEIR CAPITAL 5 5 IMPORTANT CURRENCIES 9 6 ABBREVIATIONS IN NEWS 7 LISTS OF NEW UNION COUNCIL OF MINISTERS & PORTFOLIOS 13 8 NEW APPOINTMENTS 13 9 BANK PUNCHLINES 15 10 IMPORTANT POINTS OF UNION BUDGET 2012-14 16 11 BANKING TERMS 19 12 AWARDS 35 13 IMPORTANT BANKING ABBREVIATIONS 42 14 IMPORTANT BANKING TERMINOLOGY 50 15 HIGHLIGHTS OF UNION BUDGET 2014 55 16 FDI LLIMITS 56 17 INDIAS GDP FORCASTS 57 18 INDIAN RANKING IN DIFFERENT INDEXS 57 19 ABOUT : NABARD 58 20 IMPORTANT COMMITTEES IN NEWS 58 21 OSCAR AWARD 2014 59 22 STATES, CAPITAL, GOVERNERS & CHIEF MINISTERS 62 23 IMPORTANT COMMITTEES IN NEWS 62 23 LIST OF IMPORTANT ORGANIZATIONS INDIA & THERE HEAD 65 24 LIST OF INTERNATIONAL ORGANIZATIONS AND HEADS 66 25 FACTS ABOUT CENSUS 2011 66 26 DEFENCE & TECHNOLOGY 67 27 BOOKS & AUTHOURS 69 28 LEADER”S VISITED INIDIA 70 29 OBITUARY 71 30 ORGANISATION AND THERE HEADQUARTERS 72 31 REVOLUTIONS IN AGRICULTURE IN INDIA 72 32 IMPORTANT DAMS IN INDIA 73 33 CLASSICAL DANCES IN INDIA 73 34 NUCLEAR POWER -

Impacts of International Wheat Breeding Research in Developing Countries, 1966-97
ISBN: 970-648-090-0 International Maize and Wheat Improvement Center Apdo. Postal 6-641, 06600 Mexico, D.F., Mexico www.cimmyt.org Impacts of International Wheat Breeding Research in Developing Countries, 1966-97 Paul W. Heisey, Maximina A. Lantican, and H.J. Dubin ECONOMICS PROGRAM INTERNATIONAL MAIZE AND WHEAT IMPROVEMENT CENTER (CIMMYT) CIMMYT® (www.cimmyt.org) is an internationally funded, nonprofit, scientific research and training organization. Headquartered in Mexico, CIMMYT works with agricultural research institutions worldwide to improve the productivity, profitability, and sustainability of maize and wheat systems for poor farmers in developing countries. It is one of 16 food and environmental organizations known as the Future Harvest Centers. Located around the world, the Future Harvest Centers conduct research in partnership with farmers, scientists, and policymakers to help alleviate poverty and increase food security while protecting natural resources. The centers are supported by the Consultative Group on International Agricultural Research (CGIAR) (www.cgiar.org), whose members includes nearly 60 countries, private foundations, and regional and international organizations. Financial support for CIMMYT’s research agenda also comes from many other sources, including foundations, development banks, and public and private agencies. Future Harvest® builds awareness and support for food and environmental research for a world with less poverty, a healthier human family, well-nourished children, and a better environment. It supports research, promotes partnerships, and sponsors projects that bring the results of research to rural communities, farmers, and families in Africa, Asia, and Latin America (www.futureharvest.org). International Maize and Wheat Improvement Center (CIMMYT) 2002.All rights reserved. The opinions expressed in this publication are the sole responsibility of the authors. -

Zone Wise List of NASI Fellows
The National Academy of Sciences, India (The Oldest Science Academy of India) Zone wise list of Fellows & Honorary Fellows (2021) 5, Lajpatrai Road, Prayagraj – 211002, UP, India 1 The list has been divided into six zones; and each zone is further having the list of scientists of Physical Sciences and Biological Sciences, separately. 2 The National Academy of Sciences, India 5, Lajpatrai Road, Prayagraj – 211002, UP, India Zone wise list of Fellows Zone 1 (Bihar, Jharkhand, Odisha, West Bengal, Meghalaya, Assam, Mizoram, Nagaland, Arunachal Pradesh, Tripura, Manipur and Sikkim) (Section A – Physical Sciences) ACHARYA, Damodar, Chairman, Advisory Board, SOA Deemed to be University, Khandagiri Squre, Bhubanesware - 751030; ACHARYYA, Subhrangsu Kanta, Emeritus Scientist (CSIR), 15, Dr. Sarat Banerjee Road, Kolkata - 700029; ADHIKARI, Satrajit, Sr. Professor of Theoretical Chemistry, School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja SC Mullick Road, Jadavpur, Kolkata - 700032; ADHIKARI, Sukumar Das, Formerly Professor I, HRI,Ald; Professor & Head, Department of Mathematics, Ramakrishna Mission Vivekananda University, Belur Math, Dist Howrah - 711202; BAISNAB, Abhoy Pada, Formerly Professor of Mathematics, Burdwan Univ.; K-3/6, Karunamayee Estate, Salt Lake, Sector II, Kolkata - 700091; BANDYOPADHYAY, Sanghamitra, Professor & Director, Indian Statistical Institute, 203, BT Road, Kolkata - 700108; BANERJEA, Debabrata, Formerly Sir Rashbehary Ghose Professor of Chemistry,CU; Flat A-4/6,Iswar Chandra Nibas 68/1, Bagmari Road, Kolkata - 700054; BANERJEE, Rabin, Emeritus Professor, SN Bose National Centre for Basic Sciences, Block - JD, Sector - III, Salt Lake, Kolkata - 700098; BANERJEE, Soumitro, Professor, Department of Physical Sciences, Indian Institute of Science Education & Research, Mohanpur Campus, WB 741246; BANERJI, Krishna Dulal, Formerly Professor & Head, Chemistry Department, Flat No.C-2,Ramoni Apartments, A/6, P.G. -
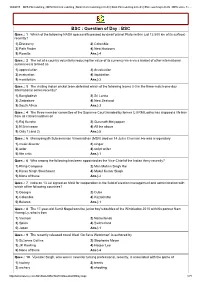
BSC : Question of Day : BSC Ques
10/8/2015 IBPS PO Coaching | IBPS PO/Clerk coaching | Bank Clerk Coaching in Delhi | Bank PO Coaching in Delhi | SSC coaching in Delhi | IBPS online Te… BSC : Question of Day : BSC Ques. : 1 Which of the following NASA spacecrafts passed by dwarf planet Pluto (within just 12,500 km of its surface) recently? 1) Discovery 2) Colombia 3) Path Finder 4) New Horizons 5) Rosetta Ans.) 4 Ques. : 2 The act of a country voluntarily reducing the value of its currency visavis a basket of other international currencies is termed as 1) appreciation 2) devaluation 3) evaluation 4) liquidation 5) moratorium Ans.) 2 Ques. : 3 The visiting Indian cricket team defeated which of the following teams 30 in the threematch oneday International series recently? 1) Bangladesh 2) Sri Lanka 3) Zimbabwe 4) New Zealand 5) South Africa Ans.) 3 Ques. : 4 The threemember committee of the Supreme Court headed by former CJI RM Lodha has slapped a life ban from all cricket matches on 1) Raj Kundra 2) Gurunath Meiyappan 3) N Srinivasan 4) All the above 5) Only 1) and 2) Ans.) 5 Ques. : 5 Manayangath Subramanian Viswanathan (MSV) died on 14 Jul in Chennai. He was a legendary 1) music director 2) singer 3) actor 4) script writer 5) film critic Ans.) 1 Ques. : 6 Who among the following has been appointed as the ViceChief of the Indian Army recently? 1) Philip Campose 2) Man Mohan Singh Rai 3) Karan Singh Shekhawat 4) Mukul Kumar Singh 5) None of these Ans.) 2 Ques. -

The Kānvar Yatrā Or Kavad Yatra Is Annual Pilgrimage of Devotees Of
P.O.BOX 5728 SRT NAGAR, NEW DELHI 110055 (BHARAT); Email : [email protected] No. Sm1407/2071 (For Private Circulation Only) Ashadha Shukla 4, Vik Samvat 2071. Yugabda 5116 Tel: 91-11- 23684445; Fax: 91-11-23517722 1 July 2014 1. FESTIVALS: The K ānvar Yatr ā or Kavad 3. HSS HINDU YOUTH CONFERENCE 2014: Yatra is annual pilgrimage of devotees of Shiva, From April 5th-6th 2014, 29 Yuvas from the known as K ānvarias who travel on foot to, NorthEast area gathered at Arsha Vidya Haridwar, Gaumukh and Gangotri in Uttarakhand Gurukulam in Saylorsburg, Pennsylvania for a to fetch holy waters of Ganges River, Ganga Jal, two-day Hindu Youth Conference. The goal of which is later offered at their local village Shiva this conference was to provide a platform for temples on the day of Shravan Shivratri or young people between the ages of 18-35 to Amavasya ( July 25 – 26 ) socialize, share ideas, and get more in touch with The Yatra used to be a small affair undertaken by their identity as Hindu Youth. Participants a few saints and old age devotees until the 1990s, included some young working professionals, and when it started gaining popularity. Today, students from various Universities like hundreds of thousands of devotees from Pennsylvania State, Drexel Rutgers etc. surrounding states of Delhi, Uttar Pradesh, Shakha was conducted in a manner geared towards Haryana, Rajasthan, Punjab and reach these places new yuva and each agnya was explained before it to participate in Kanwar Mela. was given. Many activities were planned in the The Yatra is named after the kānvar, a single pole schedule to encourage teamwork, build made of bamboo with two roughly equal loads enthusiasm, and stimulate bonding. -

Past, Present and Future
Turning research into impact: Past, present and future PROGRAM CIMMYT headquarters, El Batán, Texcoco, Estado de México Camino Real Hotel, Polanco, Mexico City 27-29 September 2016 CIMMYT - the International Maize and Wheat Improvement Center - is the global leader on publicly-funded maize and wheat research and related farming systems. Headquartered near Mexico City, CIMMYT works with hundreds of partners throughout the developing world to sustainably increase the productivity of maize and wheat cropping systems, thus improving global food security and reducing poverty. CIMMYT is a member of CGIAR and leads the CGIAR Research Programs on Maize and Wheat. The Center receives support from national governments, foundations, development banks and other public and private agencies. WELCOME For 50 years, CIMMYT has been working on the front lines of agricultural research for development, advocating for smallholder farmers and connecting national agricultural research systems to knowledge, experience, and resources. The world has changed immeasurably during this time, and CIMMYT is changing with it, but our core mission is more relevant than ever – maize and wheat scienc e for improved livelihoods. We welcome you and thank you for contributing to our mission for the last 50 years. With best regards, Martin Kropff, CIMMYT Director General All presentations will be filmed and made available on the CIMMYT50 website after the event: http://www.cimmyt.org/cimmyt50/ 1 Day 1, 27 September: Celebrating CIMMYT’s impacts CIMMYT headquarters, El Batán, -

ICAR-NDRI Annual Report 2016 17.Pdf
ANNUAL REPORT 2016-17 HkkÏvuqi&jk"Vªh; Msjh vuqlaèkku laLFkku (ekU; fo'ofo|ky;) djuky & 132 001 Hkkjr ICAR-NATIONAL DAIRY RESEARCH INSTITUTE (Deemed University) Karnal - 132 001 India ICAR-NDRI Annual Report 2016-17 © All rights reserved 2017 Publisher Dr. R. R. B. Singh Director, NDRI Chief Editor Dr. Bimlesh Mann Acting JDR, NDRI Editors Dr. Meena Malik Professor (English), NDRI Mr. Braj Kishor Assistant Chief Technical Officer, PME Layout Design, Word Processing and Cover Design Mr. Sunil Sharma Technical Officer, PME Hindi Translation Mr. Rakesh Kumar Kushwaha Assistant Director (OL) Mrs. Kanchan Choudhary Assistant Chief Technical Officer (Translator) Secretarial Operations Mr. Ram Dhari Assistant, PME Mrs. Ranjana PS to JDR Photography 2016-17 Communication Centre, NDRI T R EPO R L A NNU A RI ICAR-NDRI Annual Report is an inhouse publication. The readers are not permitted to use or sell the data, photographs and figures presented in the report. This is a report of research work carried out by the ICAR-NDRI for the year 2016-17. ICAR-ND Citation: ICAR-NDRI Annual Report 2016-17. ICAR-National Dairy Research Institute, Karnal. 234 p CONTENTS Milestones, Vision, Mission, Goal & Mandate Inside Front Cover Preface i Executive Summary iii dk;Zdkjh lkjka”k xiii 1. Introduction 1 2. Research Achievements 7 Biotechnological Interventions for Higher Productivity 8 Genetic Improvement of Dairy Animals 13 Animal Fertility, Reproduction and Diagnostics 19 Feed, Fodder and Productivity 29 Innovative Approaches in Management of Dairy Animals 36 Novel Approaches in Value Addition and Process Engineering 44 Development and Validation of Health Promoting Dairy Foods 51 Milk Proteins Derived Bioactive Peptides 63 Risk Assessment : Contaminants in Dairy Foods 69 New Generation Methods to Assess Quality and Safety of Milk and Milk Products 74 Dairy Development: Policy Analysis, Strengthening Database and Impact Assessment 78 Extension Approaches for Socio-Economic Upliftment through Dairying 81 ICT Tools and Soft Computing Applications in Dairying 88 3. -

Dr Sanjaya Rajaram 2017
Dr Sanjaya Rajaram The honorary degree of Doctor of Science in Agriculture was conferred upon Sanjaya Rajaram by the Pro-Chancellor, Mr Kevin McCann AM at a Faculty of Agriculture and Environment graduation at 2.00pm on 9 December 2017. Citation Pro-Chancellor, it gives me great pleasure to present Dr Sajaya Rajaram, an alumus of this University, to you for admission to the degree of Doctor of Science in Agriculture (honoris causa) in recognition of his exceptional achievements and eminence in the fields of international agriculture and wheat breeding, and with particularly important food security benefits to the developing world. Dr Rajaram was born in 1943 near a small farming village in the state of Uttar Pradesh in northeastern India. He completed his Bachelor of Science in Agriculture, and Masters in Wheat Breeding and Genetics in India by 1964. In 1965 he came to Australia and studied wheat breeding and genetics for his PhD at the University of Sydney, supported by a scholarship provided by the Rotary Club of Narrabri. He was located at the University’s Plant Breeding Institute in Narrabri, and his supervisors were Professor IA Watson and Nicholas Derera. He was awarded the PhD degree in 1968. He then returned to India as a Rockefeller Foundation Postdoctoral Fellow at the Indian Agricultural Research Institute in New Delhi. During this time Professor Watson recommended Dr Rajaram to the future Nobel Peace Prize Laureate Dr Norman Borlaug, who was then Wheat Director at the International Maize and Wheat Improvement Center (CIMMYT) in Mexico – and this set in motion Dr Rajaram’s distinguished scientific career in wheat research. -

The Year Book 2020
THE YEAR BOOK 2020 INDIAN ACADEMY OF SCIENCES Bengaluru Postal Address: Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post, Raman Research Institute Campus Bengaluru 560 080 India Telephone : +91-80-2266 1200, +91-80-2266 1203 Fax : +91-80-23616094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2020 Indian Academy of Sciences Information in this Year Book is updated up to 31 January 2020. Editorial & Production Team: Nalini, B.R. Thirumalai, N. Vanitha, M. Published by: Executive Secretary, Indian Academy of Sciences Text formatted by WINTECS Typesetters, Bengaluru (Ph. +91-80-2332 7311) Printed by The Print Point, Bengaluru CONTENTS Page Section A: Indian Academy of Sciences Activities – a profile ................................................................. 2 Council for the period 2019–2021 ............................................ 6 Office Bearers ......................................................................... 7 Former Presidents ................................................................... 8 The Academy Trust ................................................................. 9 Section B: Professorships Raman Chair ........................................................................... 12 Jubilee Chair ........................................................................... 15 Janaki Ammal Chair ................................................................ 16 The Academy–Springer Nature Chair ...................................... 16 Section C: