(PDE 3B) by Anthocyanin-Rich Fruit Juice Extracts and Selected Anthocyanins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Looking for New Classes of Bronchodilators
REVIEW BRONCHODILATORS The future of bronchodilation: looking for new classes of bronchodilators Mario Cazzola1, Paola Rogliani 1 and Maria Gabriella Matera2 Affiliations: 1Dept of Experimental Medicine, University of Rome Tor Vergata, Rome, Italy. 2Dept of Experimental Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy. Correspondence: Mario Cazzola, Dept of Experimental Medicine, University of Rome Tor Vergata, Via Montpellier 1, Rome, 00133, Italy. E-mail: [email protected] @ERSpublications There is a real interest among researchers and the pharmaceutical industry in developing novel bronchodilators. There are several new opportunities; however, they are mostly in a preclinical phase. They could better optimise bronchodilation. http://bit.ly/2lW1q39 Cite this article as: Cazzola M, Rogliani P, Matera MG. The future of bronchodilation: looking for new classes of bronchodilators. Eur Respir Rev 2019; 28: 190095 [https://doi.org/10.1183/16000617.0095-2019]. ABSTRACT Available bronchodilators can satisfy many of the needs of patients suffering from airway disorders, but they often do not relieve symptoms and their long-term use raises safety concerns. Therefore, there is interest in developing new classes that could help to overcome the limits that characterise the existing classes. At least nine potential new classes of bronchodilators have been identified: 1) selective phosphodiesterase inhibitors; 2) bitter-taste receptor agonists; 3) E-prostanoid receptor 4 agonists; 4) Rho kinase inhibitors; 5) calcilytics; 6) agonists of peroxisome proliferator-activated receptor-γ; 7) agonists of relaxin receptor 1; 8) soluble guanylyl cyclase activators; and 9) pepducins. They are under consideration, but they are mostly in a preclinical phase and, consequently, we still do not know which classes will actually be developed for clinical use and whether it will be proven that a possible clinical benefit outweighs the impact of any adverse effect. -

Human Erythrocyte Acetylcholinesterase in Health and Disease
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universidade de Lisboa: Repositório.UL molecules Review Human Erythrocyte Acetylcholinesterase in Health and Disease Carlota Saldanha Instituto de Bioquímica, Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Av. Professor Egas Moniz, 1649-028 Lisboa, Portugal; [email protected] Received: 10 August 2017; Accepted: 4 September 2017; Published: 8 September 2017 Abstract: The biochemical properties of erythrocyte or human red blood cell (RBC) membrane acetylcholinesterase (AChE) and its applications on laboratory class and on research are reviewed. Evidence of the biochemical and the pathophysiological properties like the association between the RBC AChE enzyme activity and the clinical and biophysical parameters implicated in several diseases are overviewed, and the achievement of RBC AChE as a biomarker and as a prognostic factor are presented. Beyond its function as an enzyme, a special focus is highlighted in this review for a new function of the RBC AChE, namely a component of the signal transduction pathway of nitric oxide. Keywords: acetylcholinesterase; red blood cells; nitric oxide 1. Introduction Erythrocytes or red blood cells (RBC) are more than sacks of oxyhemoglobin or deoxyhemoglobin during the semi-life of 120 days in blood circulation [1]. Erythrocytes comport different signaling pathways which includes the final stage of apoptosis, also called eryptosis [2,3]. Exovesicules enriched with acetylcholinesterase (AChE) originated from membranes of aged erythrocytes appear in plasma [4]. Kinetic changes of the AChE enzyme have been observed in old erythrocytes [5]. Previously, AChE in erythrocytes was evidenced as a biomarker of membrane integrity [6]. -

Cyclic Nucleotide Phosphodiesterases in Heart and Vessels
Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective Pierre Bobin, Milia Belacel-Ouari, Ibrahim Bedioune, Liang Zhang, Jérôme Leroy, Véronique Leblais, Rodolphe Fischmeister, Grégoire Vandecasteele To cite this version: Pierre Bobin, Milia Belacel-Ouari, Ibrahim Bedioune, Liang Zhang, Jérôme Leroy, et al.. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Archives of cardiovascular diseases, Elsevier/French Society of Cardiology, 2016, 109 (6-7), pp.431-443. 10.1016/j.acvd.2016.02.004. hal-02482730 HAL Id: hal-02482730 https://hal.archives-ouvertes.fr/hal-02482730 Submitted on 23 Mar 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective Abbreviated title: Cyclic nucleotide phosphodiesterases in heart and vessels French title: Phosphodiestérases des nucléotides cycliques dans le cœur et les vaisseaux : une perspective thérapeutique. Pierre Bobin, Milia Belacel-Ouari, Ibrahim Bedioune, Liang Zhang, Jérôme Leroy, Véronique Leblais, Rodolphe Fischmeister*, Grégoire Vandecasteele* UMR-S 1180, INSERM, Université Paris-Sud, Université Paris-Saclay, Châtenay-Malabry, France * Corresponding authors. UMR-S1180, Faculté de Pharmacie, Université Paris-Sud, 5 rue J.-B. Clément, F-92296 Châtenay-Malabry Cedex, France. -
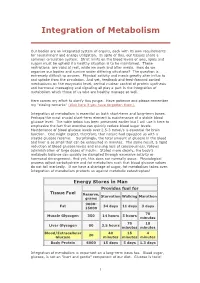
Integration of Metabolism
Integration of Metabolism Our bodies are an integrated system of organs, each with its own requirements for nourishment and energy utilization. In spite of this, our tissues share a common circulation system. Strict limits on the blood levels of ions, lipids and sugars must be upheld if a healthy situation is to be maintained. These restrictions are valid at rest, while we work and after meals. How do we organize our bodies and survive under differing situations? The question is extremely difficult to answer. Physical activity and meals greatly alter influx to and uptake from the circulation. And yet, feedback and feed-forward control mechanisms on the enzymatic level, central nuclear control of protein synthesis and hormonal messaging and signaling all play a part in the integration of metabolism which those of us who are healthy manage so well. Here comes my effort to clarify this jungle. Have patience and please remember my "closing remarks" (click here if you have forgotten them). Integration of metabolism is essential on both short-term and long-term bases. Perhaps the most crucial short-term element is maintenance of a stable blood glucose level. The table below has been presented earlier but I will use it here to emphasize the fact that exercise can quickly reduce blood sugar levels. Maintenance of blood glucose levels over 2.5-3 mmol/s is essential for brain function. One might expect, therefore, that nature had equipped us with a sizable glucose reserve. Surprisingly, the total amount of glucose in the blood and liver is so small that can be exhausted in minutes. -

Interaction of Phosphodiesterase 3A with Brefeldin A-Inhibited Guanine Nucleotide-Exchange Proteins BIG1 and BIG2 and Effect on ARF1 Activity
Interaction of phosphodiesterase 3A with brefeldin A-inhibited guanine nucleotide-exchange proteins BIG1 and BIG2 and effect on ARF1 activity Ermanno Puxeddu, Marina Uhart, Chun-Chun Li, Faiyaz Ahmad, Gustavo Pacheco-Rodriguez, Vincent C. Manganiello, Joel Moss, and Martha Vaughan1 Translational Medicine Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD 20892 Contributed by Martha Vaughan, February 11, 2009 (sent for review September 22, 2008) ADP-ribosylation factors (ARFs) have crucial roles in vesicular (11). Two-hybrid interaction of the BIG2 N-terminal region with trafficking. Brefeldin A-inhibited guanine nucleotide-exchange exocyst subunit Exo70 was confirmed by coimmunoprecipitation proteins (BIG)1 and BIG2 catalyze the activation of class I ARFs by (co-IP) of the in vitro-translated proteins; microscopically, the accelerating replacement of bound GDP with GTP. Several addi- endogenous proteins appeared together along microtubules tional and differing actions of BIG1 and BIG2 have been described. (12);3Akinase-anchoring protein (AKAP) sequences in the These include the presence in BIG2 of 3 A kinase-anchoring protein N-terminal region of BIG2, one of which is identical in BIG1, (AKAP) domains, one of which is identical in BIG1. Proteins that were identified in yeast 2-hybrid experiments with 4 different A contain AKAP sequences act as scaffolds for the assembly of PKA kinase R subunits and multiple peptide sequences representing with other enzymes, substrates, and regulators in complexes that positions 1 to 643 of BIG2 (13). Consistent with the presence of constitute molecular machines for the reception, transduction, and AKAP sequences for each of the 4 R subunits in BIG1 and BIG2, integration of signals from cAMP or other sources, which are both proteins were coimmunoprecipitated from HepG2 cell initiated, propagated, and transmitted by chemical, electrical, or cytosol with antibodies against RI or RII, although direct mechanical means. -

Diagnostic Value of Serum Enzymes-A Review on Laboratory Investigations
Review Article ISSN 2250-0480 VOL 5/ ISSUE 4/OCT 2015 DIAGNOSTIC VALUE OF SERUM ENZYMES-A REVIEW ON LABORATORY INVESTIGATIONS. 1VIDYA SAGAR, M.SC., 2DR. VANDANA BERRY, MD AND DR.ROHIT J. CHAUDHARY, MD 1Vice Principal, Institute of Allied Health Sciences, Christian Medical College, Ludhiana 2Professor & Ex-Head of Microbiology Christian Medical College, Ludhiana 3Assistant Professor Department of Biochemistry Christian Medical College, Ludhiana ABSTRACT Enzymes are produced intracellularly, and released into the plasma and body fluids, where their activities can be measured by their abilities to accelerate the particular chemical reactions they catalyze. But different serum enzymes are raised when different tissues are damaged. So serum enzyme determination can be used both to detect cellular damage and to suggest its location in situ. Some of the biochemical markers such as alanine aminotransferase, aspartate aminotransferase, alkaline phasphatase, gamma glutamyl transferase, nucleotidase, ceruloplasmin, alpha fetoprotein, amylase, lipase, creatine phosphokinase and lactate dehydrogenase are mentioned to evaluate diseases of liver, pancreas, skeletal muscle, bone, etc. Such enzyme test may assist the physician in diagnosis and treatment. KEYWORDS: Liver Function tests, Serum Amylase, Lipase, CPK and LDH. INTRODUCTION mitochondrial AST is seen in extensive tissue necrosis during myocardial infarction and also in chronic Liver diseases like liver tissue degeneration DIAGNOSTIC SERUM ENZYME and necrosis². But lesser amounts are found in Enzymes are very helpful in the diagnosis of brain, pancreas and lung. Although GPT is plentiful cardiac, hepatic, pancreatic, muscular, skeltal and in the liver and occurs only in the small amount in malignant disorders. Serum for all enzyme tests the other tissues. -

GC-MS Metabolic Profile and -Glucosidase-, -Amylase-, Lipase-, and Acetylcholinesterase-Inhibitory Activities of Eight Peach
molecules Article GC-MS Metabolic Profile and α-Glucosidase-, α-Amylase-, Lipase-, and Acetylcholinesterase-Inhibitory Activities of Eight Peach Varieties Dasha Mihaylova 1,* , Ivelina Desseva 2,* , Aneta Popova 3 , Ivayla Dincheva 4 , Radka Vrancheva 2 , Anna Lante 5 and Albert Krastanov 1 1 Department of Biotechnology, Technological Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 2 Department of Analytical Chemistry and Physical Chemistry, Technological Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 3 Department of Catering and Tourism, Economics Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 4 AgroBioInstitute, Agricultural Academy, 8 Dr. Tsankov Blvd., 1164 Sofia, Bulgaria; [email protected] 5 Department of Agronomy, Food, Natural Resources, Animals, and Environment—DAFNAE, Agripolis, University of Padova, 35020 Legnaro, Italy; [email protected] * Correspondence: [email protected] (D.M.); [email protected] (I.D.) Abstract: The inhibition of certain digestive enzymes by target food matrices represents a new approach in the treatment of socially significant diseases. Proving the ability of fruits to inhibit such enzymes can support the inclusion of specific varieties in the daily diets of patients with diabetes, obesity, Alzheimer’s disease, etc., providing them with much more than just valuable Citation: Mihaylova, D.; Desseva, I.; micro- and macromolecules. The current study aimed atidentifying and comparing the GC-MS Popova, A.; Dincheva, I.; Vrancheva, metabolic profiles of eight peach varieties (“Filina”, “Ufo 4, “Gergana”, “Laskava”, “July Lady”, R.; Lante, A.; Krastanov, A. GC-MS “Flat Queen”, “Evmolpiya”, and “Morsiani 90”) grown in Bulgaria (local and introduced) and to Metabolic Profile and α-Glucosidase-, evaluate the inhibitory potential of their extracts towards α-glucosidase, α-amylase, lipase, and α-Amylase-, Lipase-, and acetylcholinesterase. -

The Role of Phospholipase D (Pld) and Grb2 in Chemotaxis
THE ROLE OF PHOSPHOLIPASE D (PLD) AND GRB2 IN CHEMOTAXIS A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By KATIE J. KNAPEK B.S., Indiana University of Pennyslvania, 2006 2008 Wright State University WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES December 19, 2008 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISON BY Katie J. Knapek ENTITLED The Role of Phospholipase D (PLD) and Grb2 in Chemotaxis BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science. _____________________________ Julian Gomez-Cambronero, Ph. D. Thesis Director _____________________________ Barbara Hull, Ph. D. Program Director Committee on Final Examination _______________________ Julian Gomez-Cambronero, Ph. D. _______________________ Nancy Bigley, Ph. D. _______________________ Mill Miller, Ph. D. ________________________ Joseph F. Thomas Jr., Ph. D. Dean, School of Graduate Studies ABSTRACT Knapek, Katie J. M.S., Department of Biological Sciences, Microbiology and Immunology Program, Wright State University, 2008. The Role of Phospholipase D (PLD) and Grb2 in Chemotaxis. Phospholipase D (PLD) is an enzyme that hydrolyzes phosphatidylcholine yielding choline and phosphatidic acid. PLD is activated by mitogens (lead to cell division) and motogens (leading to cell migration). PLD is known to contribute to cellular proliferation and deregulated expression of PLD has been implicated in several human cancers. PLD has been found to play a role in leukocyte chemotaxis and adhesion as studied through the formation of chemokine gradients. We have established a model of cell migration comprising three cell lines: macrophages RAW 264.7 and LR-5 (for innate defense), and fibroblast COS-7 cells (for wound healing). -

Erythrocyte Acetylcholinesterase Is a Signaling Receptor
Mini Review Nov Appro Drug Des Dev - Volume 3 Issue 4 January 2018 Copyright © All rights are reserved by Carlota Saldanha DOI: 10.19080/NAPDD.2018.03.555617 Erythrocyte Acetylcholinesterase is a Signaling Receptor Carlota Saldanha* and Ana Silva Herdade Institute of Biochemistry, Institute of Molecular Medicine, Faculty of Medicine, University of Lisbon, Portugal Received Date: January 12, 2018; Published Date: January 23, 2018 *Corresponding author: Carlota Saldanha, Institute of Biochemistry, Institute of Molecular Medicine, Faculty of Medicine, University of Lisbon, Portugal, Email: Abstract The acetylcholinesterase (AChE), is an enzyme located in the erythrocyte membrane that motivate continually questions, about its physiological function. The aim of this mini review is highlight the receptor behavior of AChE in human red blood cells for chemical signals participative proteins in the steps of the signal transduction pathways, in dependence of AChE receptor, are suggested to be further considered asassociated potential with therapeutic nitric oxide targets. mobilization and efflux from erythrocytes. Data from the experimental models used are presented. Consequently, key Keywords: Erythrocyte; Acetylcholinesterase; signaling ; Acetylcholine; Receptor Abbreviations: RBCs: Red Blood Cells; AChE: Acetylcholinesterase; ACh: Acetylcholine; AC: Adenylyl cyclase; cAMP: cyclic Adenosine Monophosphate (cAMP) Mini Review The need to characterize the composition, structure and function of the red blood cells (RBCs) membrane, the presence ALS [10]. These pathologies are inflammatory vascular diseases molecules, reactive oxygen species, and reactive nitrogen species, of the acetylcholinesterase (AChE) enzyme, which kinetically characterized by high concentration in blood of inflammatory [10]. The RBCs AChE enzyme activity data obtained in those resembled the brain esterase, but markedly different from above mentioned vascular diseases reinforce the Das`statement the cholinesterase found in serum was evidenced [1]. -

Multiple Roles of Phosphoinositide-Specific Phospholipase C Isozymes
BMB reports Mini Review Multiple roles of phosphoinositide-specific phospholipase C isozymes Pann-Ghill Suh1,*, Jae-Il Park1, Lucia Manzoli2, Lucio Cocco2, Joanna C. Peak3, Matilda Katan3, Kiyoko Fukami4, Tohru Kataoka5, Sanguk Yun1 & Sung Ho Ryu1 1Division of Molecular and Life Sciences, Pohang University of Science and Technology, Pohang, Korea, 2Cellular Signaling Laboratory, Department of Anatomical Sciences, University of Bologna, Via Irnerio, 48 I-40126, Bologna, Italy, 3Cancer Research UK Centre for Cell and Molecular Biology, Chester Beatty Laboratories, The Institute of Cancer Research, Fulham Road, London SW3 6JB, UK, 4Laboratory of Genome and Biosignal, Tokyo University of Pharmacy and Life Science, 1432-1 Horinouchi, Hachioji, 192-0392 Tokyo, Japan, 5Division of Molecular Biology, Department of Biochemistry and Molecular Biology, Kobe University Graduate School of Medicine, Chuo-ku, Kobe, Japan Phosphoinositide-specific phospholipase C is an effector mole- cellular calcium release (1-3; Fig. 1a). cule in the signal transduction process. It generates two sec- The first evidence of PLC activity was suggested by Hokin et ond messengers, inositol-1,4,5-trisphosphate and diacylglycer- al. in 1953 who reported specific hydrolysis of phospholipids ol from phosphatidylinositol 4,5-bisphosphate. Currently, thir- in pigeon’s pancreas slices after cholinergic stimulation (4). teen mammal PLC isozymes have been identified, and they are The authors showed that the enhanced turnover of phosphor- divided into six groups: PLC-β, -γ, -δ, -ε, -ζ and -η. Sequence ylinositol groups of phosphatidylinositol occurred in cells as a analysis studies demonstrated that each isozyme has more response to a variety of stimuli. In 1983, Streb et al. -

Supplementary Table 2
Supplementary Table 2. Differentially Expressed Genes following Sham treatment relative to Untreated Controls Fold Change Accession Name Symbol 3 h 12 h NM_013121 CD28 antigen Cd28 12.82 BG665360 FMS-like tyrosine kinase 1 Flt1 9.63 NM_012701 Adrenergic receptor, beta 1 Adrb1 8.24 0.46 U20796 Nuclear receptor subfamily 1, group D, member 2 Nr1d2 7.22 NM_017116 Calpain 2 Capn2 6.41 BE097282 Guanine nucleotide binding protein, alpha 12 Gna12 6.21 NM_053328 Basic helix-loop-helix domain containing, class B2 Bhlhb2 5.79 NM_053831 Guanylate cyclase 2f Gucy2f 5.71 AW251703 Tumor necrosis factor receptor superfamily, member 12a Tnfrsf12a 5.57 NM_021691 Twist homolog 2 (Drosophila) Twist2 5.42 NM_133550 Fc receptor, IgE, low affinity II, alpha polypeptide Fcer2a 4.93 NM_031120 Signal sequence receptor, gamma Ssr3 4.84 NM_053544 Secreted frizzled-related protein 4 Sfrp4 4.73 NM_053910 Pleckstrin homology, Sec7 and coiled/coil domains 1 Pscd1 4.69 BE113233 Suppressor of cytokine signaling 2 Socs2 4.68 NM_053949 Potassium voltage-gated channel, subfamily H (eag- Kcnh2 4.60 related), member 2 NM_017305 Glutamate cysteine ligase, modifier subunit Gclm 4.59 NM_017309 Protein phospatase 3, regulatory subunit B, alpha Ppp3r1 4.54 isoform,type 1 NM_012765 5-hydroxytryptamine (serotonin) receptor 2C Htr2c 4.46 NM_017218 V-erb-b2 erythroblastic leukemia viral oncogene homolog Erbb3 4.42 3 (avian) AW918369 Zinc finger protein 191 Zfp191 4.38 NM_031034 Guanine nucleotide binding protein, alpha 12 Gna12 4.38 NM_017020 Interleukin 6 receptor Il6r 4.37 AJ002942 -

Pancreatic Amylase and Lipase Plasma Concentrations Are
Diabetes Care Volume 38, May 2015 e71 Pancreatic Amylase and Lipase Plasma David P. Sonne,1 Tina Vilsbøll,1 and Filip K. Knop1,2 Concentrations Are Unaffected by Increments in Endogenous GLP-1 Levels Following Liquid Meal Tests Diabetes Care 2015;38:e71–e72 | DOI: 10.2337/dc14-2751 Recent findings have suggested that amylase and lipase in plasma following patients with type 2 diabetes (P , incretin-based therapies promote pan- the ingestion of oral glucose and three 0.05), whereas lipase concentrations creatic inflammation and possibly cell isocaloric and isovolemic liquid mealsd were similar. Neither of the enzymes proliferation within the endocrine and all of which exerted normal endogenous increased following nutrient ingestion, exocrine pancreas (1). However, these GLP-1 secretion (4)din patients with suggesting that postprandial elevations studies have been met with substan- type 2 diabetes and matched control of endogenous GLP-1 (two- to three- tive criticism based on technical and subjects. fold) cannot trigger enzyme release methodical issues (2). Nevertheless, Detailed description of the experimen- from the human pancreas, at least not incretin-based therapies seem to result tal procedures and subjects was provided acutely. in small increases (within normal range) previously (4). In short, pancreas-specific These results suggest that the obser- in plasma concentrations of amylase amylase and lipase concentrations were vation of elevated plasma amylase and andlipaseinpatientsreceivingthese measured in plasma from 15 patients lipase