Evaluation of Anti-Corrosion and Anti-Galling Performance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improvement of High−Temperature Characteristics of the Sintered
686 EJ60ケooho勿zゑsψりノ 一Article一 Improvement of High-Temperature Characteristics of the Sintered Nickel Positive Electrode for an Alkaline Storage Battery Katsuhiko SHINYAMA,*Yoshifumi MAGARI,Atsuhiro FUNAHASHI, Toshiyuki NOHMA,and Ikuo YONEZU R&D Business Unit,Mobile Energy Company,Sanyo Electric Co.,Ltd.(7-3-21bukidai-higashimachi,Nishi-ku,Kobe City,Hyogo651-2242,Japan) Received August9,2002;Accepted May20,2003 The high-temperature characteristics of sintered nickel positive electrodes for alkaline storage batteries such as nickel・metal hydride batteries and nicke1-cadmium batteries were investigated.Generally,the discharge capacity of an alkaline storage battery charged at high temperature is smaller than that charged at room temperature due to the oxygen evolution reaction.In order to enhance high-temperature characteristics,we coated sintered n量ckel positive electrodes with yttrium hydroxide,calcium hydroxide or cobalt hydroxide.Th6high-temperature characteristics of the sintered nickel positive electrodes coated with y血ium hydroxide and calcium hydroxide by immersing them in sodium hydroxide solution after immersing in nitrate solution were greatly enhanced because of the increased oxy. gen OVerVOltage. κ¢y防鳩:Sintered Nickel Positive Electrode,High-temperature Characteristics,Alkaline Storage Battery,Oxygen Overvoltage l lntroduction In the past,the electrolyte composition3)and dissolv- Cun℃ntly,alkaline storage batteries such as nicke1- ing such elements as cobalt and calcium into nickel hy- cadmium batteries and nickel-metal hydride -

Thread Galling: What Is It & How to Prevent It?
Thread Galling: What Is It & How to Prevent It? Thread galling is a The Culprits: Metal Fasteners common yet a This generally happens to metal fasteners seldom understood when their protective oxide layer is worn fastener problem away during the installation process due seen by engineers to the increased pressure and heat and fabricators. It between the contacting thread surfaces. occurs when With the absence of the oxide coating, pressure and friction cause bolt the raw metal threads contact each other threads to seize to the threads of a nut directly with no barrier, causing the the or tapped hole. The fasteners are not nut and bolt to “fuse” together. Prior to just overly tight but “cold/contact” use, you inspect all fasteners, yet after welded together. installation they still fail. So how do you prevent this? Galling Prevention Install Slowly and Correctly Slowing down the installation RPM speed can often reduce and even completely prevent galling issues. As the installation speed increases, so does friction – resulting in the heat production that can cause galling. Also, over-tightening can also increase friction and galling. Use Lubrication and Coatings When Possible Lubricating the internal and/or external threads reduces friction which is a primary element of galling. Always take into consideration the end use of the fasteners before selecting a lubricant. Applications where food or laboratory purity is a concern may make some lubricants unacceptable. In addition, the fastener installer must take into consideration changes in the torque-tension relationship due to the use of lubricants as over-tightening can also result in galling. -

Rapid Coprecipitation Technique Using Yttrium Hydroxide for the Preconcentration and Separation of Trace Elements in Saline Water Prior to Their ICP-AES Determination
ANALYTICAL SCIENCES AUGUST 2007, VOL. 23 1021 2007 © The Japan Society for Analytical Chemistry Notes Rapid Coprecipitation Technique Using Yttrium Hydroxide for the Preconcentration and Separation of Trace Elements in Saline Water Prior to Their ICP-AES Determination Shigehiro KAGAYA,† Satoshi MIWA, Toshiyuki MIZUNO, and Koji TOHDA Graduate School of Science and Engineering for Research, University of Toyama, Gofuku 3190, Toyama 930–8555, Japan Yttrium hydroxide quantitatively coprecipitated Be(II), Ti(IV), Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Cd(II), and Pb(II) at pH 9.6 – 10.0 for seawater and pH 10.5 – 11.4 for a table-salt solution. The coprecipitated elements could be determined by inductively coupled plasma atomic emission spectrometry; yttrium was used as an internal standard element. The detection limits ranged from 0.0016 μg (Mn(II)) to 0.22 μg (Zn(II)) in 100 mL of sample solutions. The operation time required to separate 11 elements was approximately 30 min. (Received May 25, 2007; Accepted June 18, 2007; Published August 10, 2007) It is important to determine trace elements in environmental of many elements. However, to our knowledge, no application samples from the viewpoint of environmental management and/or of yttrium hydroxide to the preconcentration/separation of trace protection from hazardous infection1 as well as comprehension elements in environmental water samples has been reported. of the distribution of trace elements in the environment.2 In this work, we developed a preconcentration/separation Recently, multielement profiling analysis, in which major-to- method based on a rapid coprecipitation technique using yttrium ultratrace elements are determined and the distribution patterns hydroxide as a coprecipitant for the determination of trace of the elements are analyzed, has gathered attention to provide elements in environmental water samples, especially saline information about the elemental cycle in the environment.3,4 water. -
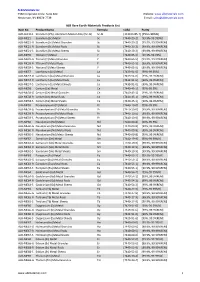
ALB Materials Inc Product List.Xlsx
ALB Materials Inc 2360 Corporate Circle. Suite 400 Website: www.albmaterials.com Henderson, NV 89074-7739 E-mail: [email protected] ALB Rare Earth Materials Products List Item No. Product Name Formula CAS# Purity ALB-AL2113 Scandium (2%)- Aluminum Master Alloy (Sc-Al) Sc-Al [113413-85-7] [2%Sc+98%Al] ALB-ME21 Scandium (Sc) Metal Sc [7440-20-2] [99.9%-99.999%] ALB-ME21-G Scandium (Sc) Metal Granules Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME21-R Scandium (Sc) Metal Rods Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME21-S Scandium (Sc) Metal Sheets Sc [7440-20-2] [99.9%, 99.99%REM] ALB-ME39 Yttrium (Y) Metal Y [7440-65-5] [99.9%-99.99%] ALB-ME39-G Yttrium (Y) Metal Granules Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME39-R Yttrium (Y) Metal Rods Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME39-S Yttrium (Y) Metal Sheets Y [7440-65-5] [99.9%, 99.99%REM] ALB-ME57 Lanthanum (La) Metal La [7439-91-0] [99%-99.95%] ALB-ME57-G Lanthanum (La) Metal Granules La [7439-91-0] [99%, 99.9%REM] ALB-ME57-R Lanthanum (La) Metal Rods La [7439-91-0] [99%, 99.9%REM] ALB-ME57-S Lanthanum (La) Metal Sheets La [7439-91-0] [99%, 99.9%REM] ALB-ME58 Cerium (Ce) Metal Ce [7440-45-1] [99%-99.9%] ALB-ME58-G Cerium (Ce) Metal Granules Ce [7440-45-1] [99%, 99.9%REM] ALB-ME58-R Cerium (Ce) Metal Rods Ce [7440-45-1] [99%, 99.9%REM] ALB-ME58-S Cerium (Ce) Metal Sheets Ce [7440-45-1] [99%, 99.9%REM] ALB-ME59 Praseodymium (Pr) Metal Pr [7440-10-0] [99%-99.9%] ALB-ME59-G Praseodymium (Pr) Metal Granules Pr [7440-10-0] [99.9%, 99.99%REM] ALB-ME59-R Praseodymium (Pr) Metal Rods Pr [7440-10-0] -

Surface Finishing Treatments
Tel.: 1-800-479-0056 Fax: 1-888-411-2841 www.aspenfasteners.com [email protected] Surface finishing treatments Surface finishing treatments can have a significant impact on the properties of fasteners, making them more suitable for specific applications. Most typically finishes are applied to improve durability (wear resistance and corrosion resistance) and/or for decorative purposes. Fasteners that are to be used in conditions where they are exposed to high physical stress levels, corrosive elements, or extreme temperatures may benefit greatly from plating/coating. Anodizing An electrolytic passivation process that forms a thin, transparent oxide layer that protects the metal substrate. Anodizing modifies the surface of the metal to produce a decorative, durable, corrosion and wear resistant finish. Unlike plating or painting, the oxide finish becomes integrated with the metallic substrate, so it cannot chip or peel and minimally alters the dimensional aspects of the part. The physical properties of this oxide layer also provides excellent adhesion for secondary processes like colouring and sealing. The term "anodizing" reflects the process whereby the metal part to be finished forms the anode of an electric circuit immersed in an electrolyte bath. The current in the circuit causes ionic oxygen to be released from the electrolyte and combine with the metallic substrate of the finished part. Black Oxide Black Oxide is a low cost conversion coating where oxidizing salts are used to react with the iron in steel alloys to form magnetite (Fe304), the black oxide of iron. The result is an attractive and durable matte black finish. While steel is the usual substrate, other materials including stainless steel, alloy, copper, brass, bronze, die cast zinc, and cast iron react equally well. -

Zirconia-Ceria-Yttria-Based Mixed Oxide and Process for Producing The
(19) TZZ_ _Z_T (11) EP 1 921 044 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C01G 25/00 (2006.01) B01J 23/10 (2006.01) 28.02.2018 Bulletin 2018/09 B01D 53/94 (2006.01) B01J 21/06 (2006.01) B01J 35/10 (2006.01) (21) Application number: 07118246.3 (22) Date of filing: 10.10.2007 (54) Zirconia-ceria-yttria-based mixed oxide and process for producing the same Auf Zirkonium-Cerium-Yttrium basierendes Mischoxid und Herstellungsverfahren dafür Oxyde mixte à base de zirconium-cérium-yttrium et son procédé de fabrication (84) Designated Contracting States: • Kodama, Hiroshi DE FR GB Osaka 5590025 (JP) (30) Priority: 12.10.2006 JP 2006305934 (74) Representative: Manley, Nicholas Michael 08.08.2007 JP 2007206394 WP Thompson 8th Floor (43) Date of publication of application: 1 Mann Island 14.05.2008 Bulletin 2008/20 Liverpool L3 1BP (GB) (73) Proprietor: Daiichi Kigenso Kagaku Kogyo Co., (56) References cited: Ltd. EP-A- 1 006 081 EP-A- 1 035 074 Osaka-shi, EP-A- 1 894 620 WO-A-2007/093593 Osaka 559-0025 (JP) JP-A- 2003 277 059 US-B1- 6 387 338 (72) Inventors: • Okamoto, Hiroshi Osaka 5590025 (JP) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. Notice of opposition shall not be deemed to have been filed until the opposition fee has been paid. -

A Crystal Plasticity Assessment of Normally-Loaded Sliding Contact in Rough Surfaces and Galling
A Crystal Plasticity Assessment of Normally-loaded Sliding Contact in Rough Surfaces and Galling Bartosz Barzdajna,1,*, Anthony T. Paxtonb, David Stewartc, Fionn P.E. Dunnea aDepartment of Materials, Imperial College, London SW67 2AZ, UK bDepartment of Physics, King’s College London, London WC2R 2LS, UK cRolls-Royce plc, Raynesway, Derby DE21, UK 1Now at School of Materials, The University of Manchester, Manchester M13 9PL, UK Abstract An investigation of rough metal to metal contacting surfaces under normal load and undergoing sliding has been carried out with explicit representation of measured surface profiles within a crystal plasticity finite element formulation in which grain size, texture and slip properties are incorporated. A new metric called plastic reach has been introduced for contacting surfaces which reflects both the magnitude of the local surface asperity plasticity and its spatial reach. This quantity has been shown to obey a power law relationship with the applied normal load for sliding contact which in turn has been related to a hazard function. In this way, a new methodology to predict the galling frequency that follows a Weibull distribution has been established. Additionally, a quantitative definition of galling for the class of metal on metal contacting surfaces is considered. The predicted galling frequency distribution for a 316 stainless steel has been compared with independently experimentally measured galling frequencies showing qualitative agreement of the distributions. An assessment of confidence limits has also therefore been provided for the modelling methodology. Keywords: galling, rough surfaces, sliding contact, crystal plasticity * Corresponding author: [email protected] 1 1. Introduction This paper addresses the problem of galling of metallic materials. -

Copper Alloys ______
COPPER ALLOYS ___________________________ Guidelines for the use of copper alloys ·in seawater* Arthur H. Tuthill* rate of film formation is indicated as the rate of copper reduction in the Users and designers have found the charts and data sum effluent (Figure 1). Total copper decreases tenfold within 10 min and maries in an earlier work, " Guidelines for Selection of Marine 100-fold in the first hour. In three months, copper in the effluent is Materials," helpful in selecting materials for marine service. seen to be virtually at the level of the copper in the intake water. This review updates and extends those summaries for copper-base alloys, many of which were not included in the Weight loss corrosion studies show that the protective film earlier work. continues to improve, with the corrosion rate dropping to 0.5 mpy (0.012 mm/y) in - 1 y, and a long-term, steady-state rate of - 0.05 mpy (0.001 mm/y) in 3 to 7 y in quiet, tidal, and flowing seawater (Figure 2). 2 Alloy C71500 (70 :30 copper-nickel) exhibits the same pattern of decreasing corrosion rate with time. Introduction Reinhart found that copper and its alloys of aluminum, silicon, THE ENGINEER USUALLY BEGINS WITH a good idea of alloys that tin , beryllium, and nickel had significantly lower long-term corrosion will meet the stresses and mechanical requirements of the assembly rates after 18 months compared to those specimens measured after under consideration. The author's purpose is to provide guidelines only 6 months of exposure to seawater (Figure 3).3 The only that will allow the engineer to make a reasonable estimate of the exception was Alloy C28000, Muntz metal, which exhibited a slightly effect of the environment on the performance of copper alloys. -

Enghandbook.Pdf
785.392.3017 FAX 785.392.2845 Box 232, Exit 49 G.L. Huyett Expy Minneapolis, KS 67467 ENGINEERING HANDBOOK TECHNICAL INFORMATION STEELMAKING Basic descriptions of making carbon, alloy, stainless, and tool steel p. 4. METALS & ALLOYS Carbon grades, types, and numbering systems; glossary p. 13. Identification factors and composition standards p. 27. CHEMICAL CONTENT This document and the information contained herein is not Quenching, hardening, and other thermal modifications p. 30. HEAT TREATMENT a design standard, design guide or otherwise, but is here TESTING THE HARDNESS OF METALS Types and comparisons; glossary p. 34. solely for the convenience of our customers. For more Comparisons of ductility, stresses; glossary p.41. design assistance MECHANICAL PROPERTIES OF METAL contact our plant or consult the Machinery G.L. Huyett’s distinct capabilities; glossary p. 53. Handbook, published MANUFACTURING PROCESSES by Industrial Press Inc., New York. COATING, PLATING & THE COLORING OF METALS Finishes p. 81. CONVERSION CHARTS Imperial and metric p. 84. 1 TABLE OF CONTENTS Introduction 3 Steelmaking 4 Metals and Alloys 13 Designations for Chemical Content 27 Designations for Heat Treatment 30 Testing the Hardness of Metals 34 Mechanical Properties of Metal 41 Manufacturing Processes 53 Manufacturing Glossary 57 Conversion Coating, Plating, and the Coloring of Metals 81 Conversion Charts 84 Links and Related Sites 89 Index 90 Box 232 • Exit 49 G.L. Huyett Expressway • Minneapolis, Kansas 67467 785-392-3017 • Fax 785-392-2845 • [email protected] • www.huyett.com INTRODUCTION & ACKNOWLEDGMENTS This document was created based on research and experience of Huyett staff. Invaluable technical information, including statistical data contained in the tables, is from the 26th Edition Machinery Handbook, copyrighted and published in 2000 by Industrial Press, Inc. -

Tompbnents of Fission and 'Natural Radioactivity Found in Water Are
DOCUMENT RESUME,, A ED '170 1110 SE027 bill. 0 AUTHOR Thatcher, L.L. ;And Others 1 TITLE Techniques of Water-Resources Investigations of the United. States' Geblogical.Survey. Book 5, Laboratory, Analysis. Chapter- A5, Methodscr Dete-rmination of - Radioactive Substances in Water and Fluvial Sediments. INSTITUTION -Geological Survey (Dept,: .of Interior) ,Reston, PUB DATE 77 NO Tr 95p. ;For related document, see ED 131 218 AVAILABLE FRQM Super.intendent of Documents, U.S. Government Printing Off /ce , Washington, D.C. -20402(Stock Number 024- 0.01- 02928- 6:, No price quoted) */ FDRS PRICE MF01/PC04 Plus Postage. DESCRIPTORS *ChemiCal Analysis; *Geolo'gy; *LaboratorTechniques; PhYsic; Pollution; Retource Materials; S.c ence Equipment; Water Pollution control IDENTIFIERS *RadioactiveVs- Substances; *Sediments; Water Quality ABSTkACT Analytical methods for determining important , tompbnents of fission and 'natural radioactivity found in water are reportedThe discussion of each method includes conditions for application of the method, a summary of the method, interferences, required apparatus, Procedures, calculations and estimation of precision. Isotopes considered are cesium-137, strontium-90, lead-210, radium-226. and 228, tritium, and carbon-714. `When two methods are in use for an isotope,- both methods are reported. Techniques for the collection and preservation of water samples to be anallze?d for radioactivity are also discuSsed.(CS) v. *********************************************************************** 4 Re. productions supplied -

Interagency Committee on Chemical Management
DECEMBER 14, 2018 INTERAGENCY COMMITTEE ON CHEMICAL MANAGEMENT EXECUTIVE ORDER NO. 13-17 REPORT TO THE GOVERNOR WALKE, PETER Table of Contents Executive Summary ...................................................................................................................... 2 I. Introduction .......................................................................................................................... 3 II. Recommended Statutory Amendments or Regulatory Changes to Existing Recordkeeping and Reporting Requirements that are Required to Facilitate Assessment of Risks to Human Health and the Environment Posed by Chemical Use in the State ............................................................................................................................ 5 III. Summary of Chemical Use in the State Based on Reported Chemical Inventories....... 8 IV. Summary of Identified Risks to Human Health and the Environment from Reported Chemical Inventories ........................................................................................................... 9 V. Summary of any change under Federal Statute or Rule affecting the Regulation of Chemicals in the State ....................................................................................................... 12 VI. Recommended Legislative or Regulatory Action to Reduce Risks to Human Health and the Environment from Regulated and Unregulated Chemicals of Emerging Concern .............................................................................................................................. -

Galling Resistance of Toughmet 2 Alloy and Two Other Copper Alloys Were Measured Against 4140 Steel
TECH BRIEF GALLING RESISTANCE OF TOUGHMET® 2 ALLOY AND OTHER COPPER ALLOYS The galling resistance of ToughMet 2 alloy and two other copper alloys were measured against 4140 steel. ToughMet 2 alloy had the greatest deformation resistance of the three non-galling alloys. INTRODUCTION 4140 steel with one of the copper alloys as the button with- out lubrication. Stress was applied using an Instron Mechanical The galling resistance and galling threshold stress of three cop- Testing Unit beginning with increments of 10 ksi (69 MPa). Visual per-based alloys was measured against 4140 steel tempered to examination of the copper buttons was performed to deter- HRC 35. These tests were conducted to provide information mine if a button had galled or deformed. Galling resistance is on the relative value of ToughMet 2 alloy in galling applications reported as the lowest stress at which neither galling or button such as wear plates and bushings. The copper alloys chosen for deformation was observed. comparative tests, C95400 and C95900, are alloys commonly used in these applications. RESULTS AND DISCUSSION The test method employed for the measurement, ASTM G98, Galling of the copper alloys to the 4140 steel was not observed provides a threshold value below which galling will not occur. in the tests prior to deformation of the buttons. The failure Many wear plate designs incorporate lubrication such as graph- criterion for the tests, therefore, was not galling but the stress ite or graphite plugs. Ultimately, the failure mechanism of these at which deformation was observed. These results are listed in plates, or bushings, is adhesive wear of the copper alloy, which Table 1.