+ Oxygen Gas CO2 (G) + H2O (G) D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary-Explosives
Improvised Primary Explosives © 1998 Dirk Goldmann No part of the added copyrighted parts (except brief passages that a reviewer may quote in a review) may be reproduced in any form unless the reproduced material includes the following two sentences: The copyrighted material may be reproduced without obtaining permission from anyone, provided: (1) all copyrighted material is reproduced full-scale. WARNING! Explosives are danegerous. In most countries it's forbidden to make them. Use your mind. You as an explosives expert should know that. 2 CONTENTS Primary Explosives ACETONE PEROXIDE 4 DDNP/DINOL 6 DOUBLE SALTS 7 HMTD 9 LEAD AZIDE 11 LEAD PICRATE 13 MEKAP 14 MERCURY FULMINATE 15 "MILK BOOSTER" 16 NITROMANNITE 17 SODIUM AZIDE 19 TACC 20 Exotic and Friction Primers LEAD NITROANILATE 22 NITROGEN SULFIDE 24 NITROSOGUANIDINE 25 TETRACENE 27 CHLORATE-FRICTION PRIMERS 28 CHLORATE-TRIMERCURY-ACETYLIDE 29 TRIHYDRAZINE-ZINC (II) NITRATE 29 Fun and Touch Explosives CHLORATE IMPACT EXPLOSIVES 31 COPPER ACETYLIDE 32 DIAMMINESILVER II CHLORATE 33 FULMINATING COPPER 33 FULMINATING GOLD 34 FULMINATING MERCURY 35 FULMINATING SILVER 35 NITROGEN TRICHLORIDE 36 NITROGEN TRIIODIDE 37 SILVER ACETYLIDE 38 SILVER FULMINATE 38 "YELLOW POWDER" 40 Latest Additions 41 End 3 PRIMARY EXPLOSIVES ACETONE PEROXIDE Synonyms: tricycloacetone peroxide, acetontriperoxide, peroxyacetone, acetone hydrogen explosive FORMULA: C9H18O6 VoD: 3570 m/s @ 0.92 g/cc. 5300 m/s @ 1.18 g/cc. EQUIVALENCE: 1 gram = No. 8 cap .75 g. = No. 6 cap SENSITIVITY: Very sensitive to friction, flame and shock; burns violently and can detonate even in small amounts when dry. DRAWBACKS: in 10 days at room temp. 50 % sublimates; it is best made immediately before use. -

Safety Data Sheet
SAFETY DATA SHEET According to JIS Z 7253:2019 Revision Date 28-Oct-2020 Version 3.02 Section 1: PRODUCT AND COMPANY IDENTIFICATION Product name Silver Chlorate Product code 192-05752,194-05751 Manufacturer FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-5964 Supplier FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome, Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-2029 Emergency telephone number +81-6-6203-3741 / +81-3-3270-8571 Recommended uses and For research purposes restrictions on use Section 2: HAZARDS IDENTIFICATION GHS classification Classification of the substance or mixture Oxidizing solids Category 2 Acute toxicity - Oral Category 3 Pictograms Signal word Danger Hazard statements H272 - May intensify fire; oxidizer H301 - Toxic if swallowed Precautionary statements-(Prevention) • Keep away from heat/sparks/open flames/hot surfaces. — No smoking • Keep/Store away from clothing/combustible materials • Wear protective gloves/protective clothing/eye protection/face protection • Wash face, hands and any exposed skin thoroughly after handling • Do not eat, drink or smoke when using this product • Take any precaution to avoid mixing with combustibles Precautionary statements-(Response) • Wash contaminated clothing before reuse. • IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician • Rinse mouth. Precautionary statements-(Storage) • Store locked up. Precautionary statements-(Disposal) • Dispose of contents/container to an approved waste disposal plant __________________________________________________________________________________________ Page 1 / 6 W01W0119-0575 JGHEEN Silver Chlorate Revision Date 28-Oct-2020 __________________________________________________________________________________________ Others Other hazards Not available Section 3: COMPOSITION/INFORMATION ON INGREDIENTS Single Substance or Mixture Substance Formula AgClO3 Chemical Name Weight-% Molecular weight ENCS ISHL No. -

DYNAMAX 240 DOT 3 Brake Fluid
Previous version preparation date: 23.10.2013 Page: 1/7 Update date: 12.09.2017 Revision edition no: 2 DYNAMAX 240 DOT 3 Brake fluid Safety data sheet (Regulation 1907/2006/EC, amended by 2015/830/EU) SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY / UNDERTAKING 1.1. Product identifier Material Name: DYNAMAX 240 DOT 3 CAS: EINECS: ELINCS: 1.2. Identified uses Uses: Brake fluid 1.3. Details of the supplier of the safety data sheet Manufacturer/Supplier: EURO-VAT spol. s r.o. IČO: 18049397 Address: Alekšince 231 951 22 Alekšince (Slovakia) Phone/fax number: +421 37 7822 326-7 E-mail: [email protected] www.eurovat.sk Emergency phone Národné toxikologické informa čné centrum (NTIC) number: Klinika pracovného lekárstva a toxikológie, FNsP akad. L.Dérera Limbova 5 833 05 Bratislava Tel. +421 2 5477 4166 [email protected] www.ntic.sk SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the Classification according to Regulation (EC) No. 1272/2008 [CLP/GHS] substance or mixture Eye Dam. 1 H318 Hazard pictograms (CLP/GHS) : 2.2 Label elements Signal word: danger H-phrases: H318 Causes serious eye damage EUH208 Contains 2,4-dimethyl-6-tert-butyl phenol. May produce an allergic reaction. P-phrases: P102 Keep out of reach of children P280 Wear protective gloves/protective clothing/eye protection/face protection P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing P310 Immediately call a POISON CENTER/doctor/. Contains: 2-[2(2-buthoxy ethoxy)ethoxy]ethanol Previous version preparation date: 23.10.2013 Page: 2/7 Update date: 12.09.2017 Revision edition no: 2 DYNAMAX 240 DOT 3 Brake fluid Precautionary statements Prevention : Do not breathe vapour. -

Oxidant Generation on Photolysis of Silver Chloride Suspensions: Implications to Organic Contaminant
Oxidant generation on photolysis of silver chloride suspensions: implications to organic contaminant degradation Tian Ma Supervisor: Scientia Professor T. David Waite A thesis in fulfilment of the requirements for the degree of Doctor of Philosophy School of Civil and Environmental Engineering Faculty of Engineering March, 2014 A ORIGINALITY STATEMENT ‘I hereby declare that this submission is my own work and to the best of my knowledge it contains no materials previously published or written by another person, or substantial proportions of material which have been accepted for the award of any other degree or diploma at UNSW or any other educational institution, except where due acknowledgement is made in the thesis. Any contribution made to the research by others, with whom I have worked at UNSW or elsewhere, is explicitly acknowledged in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in the project's design and conception or in style, presentation and linguistic expression is acknowledged.’ Signed …………………………………………….............. Date ……………………….............................................. i COPYRIGHT STATEMENT ‘I hereby grant the University of New South Wales or its agents the right to archive and to make available my thesis or dissertation in whole or part in the University libraries in all forms of media, now or here after known, subject to the provisions of the Copyright Act 1968. I retain all proprietary rights, such as patent rights. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. -

Mono Ethylene Glycol (Meg)
PRODUCT INFORMATION MONO ETHYLENE GLYCOL (MEG) Chemical Formula: C2H6O2 CAS Registry Number: 107‐21‐1 Molecular Weight: 62.068 Synonyms: • 1,2-Dihydroxyethane • 1,2-Ethane Diol • 1,2-Ethanediol • 1,2-Ethylene glycol • 146AR • 2-Hydroxyethanol • 48: PN: WO2006074281 PAGE: 43 claimed sequence • AETHYLENGLYKOL • DIHYDROXY ETHANE, 1,2- (FIBER GRADE) • Dowtherm SR 1 • E 600 • E 600 (glycol) • etano-1,2-diol • Ethan-1,2-diol • Ethane-1,2-diol • Ethylene alcohol • Ethylene dihydrate • Ethylene glycol • ETHYLENE GLYCOL, MONO (FIBER GRADE) • ETHYLENEGLYCOL • Fridex • Glycol • GLYCOL (FIBER GRADE) • Glycol alcohol • Glysil GS ======================================================================================== • Macrogol 400 BPC NOTICE: THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST • MEG 100 INFORMATION CURRENTLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF • MONOETHYLENE GLYCOL MERCHANTABILITY OR ANY OTHER WARRANTY, EXPRESS OR IMPLIED, WITH RESPECT TO SUCH • Norkool INFORMATION, AND WE ASSUME NO LIABILITY RESULTING FROM ITS USE. USERS SHOULD MAKE THEIR • NSC 93876 OWN INVESTIGATIONS TO DETERMINE THE SUITABILITY OF THE INFORMATION FOR THEIR PARTICULAR • POLYESTER OF 1,2- PURPOSES. IN NO EVENT SHALL SAMCHEM PRASANDHA BE LIABLE FOR ANY CLAIMS, LOSSES, OR ETHANEDIOL DAMAGES OF ANY THIRD PARTY OR LOST PROFITS OR ANY SPECIAL, INDIRECT, INCIDENTAL, CONSEQUENTIAL OR EXEMPLARY DAMAGES, HOWSOEVER ARISING, EVEN IF SAMCHEM PRASANDHA HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. ====================================================================== PT. Samchem Prasandha Taman Palem Lestari Block A30 No. 26 www.samchemprasandha.com Cengkareng ‐ Jakarta 11730 ‐ Indonesia Phone : 62‐21‐55952991 (hunting) Page | 1 Fax : 62‐21‐55952994 Physical and Chemical Properties Appearance: Clear oily liquid. Odor: Odorless. Solubility: Miscible in water. Specific Gravity: 1.1 @20°C/4°C pH: No information found. -

GRAS Notification for Chlorine Dioxide and Withdrawal of FAP 2266; Resonant Biosciences, Inc.; Our File No
I® ORIGINAL 1001 G Street, N.W. Suite 500 West Washington, D.C. 20001 tel. 202.434.4100 fax 202.434.4646 Writer's Direct Access Martha E. Marrapese (202) 434-4 123 November 11. 2010 [email protected] Via Federal Express CONTAINS CONFIDENTIAL BUSINESS INFORMATION Food and Drug Administration Division of Animal Feeds (HFV-224) Office of Surveillance and Compliance Center for Veterinary Medicine 7519 Standish Place Rockville, Maryland 20855 Re: GRAS Notification for Chlorine Dioxide and Withdrawal of FAP 2266; Resonant Biosciences, Inc.; Our File No. RE14070 Dear Sir or Madam: The purpose of this letter is to request to participate in the pilot program for a Generally Recognized as safe (GRAS) determination for the safe use of chlorine dioxide generated by the notifier's PureMash* system in the production of food grade and non-food grade ethanol and distillers grains for animal feed use (food producing livestock). 75 Fed. Reg. 31800 (June 4, 2010). In connection with this GRAS notification, Resonant Biosciences, Inc. (RBS or Notifier) is formally withdrawing Food Additive Petition (FAP) 2266. The Notifier previously met with your office to discuss residual testing and a submission plan for this product. The potential eligibility for clearance under the Federal Food, Drug, and Cosmetic Act (FFDCA) as GRAS was raised at that time. We trust that our prior discussions fully satisfy the guidance in the Federal Register that "FDA strongly encourages potential participants in the animal food pilot program to contact the Division of Animal Feeds" prior to submitting notices. 75 Fed. Reg. at 31802. We look forward to confirmation that this submission has been accepted and is complete. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -
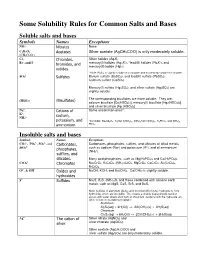
Some Solubility Rules for Common Salts and Bases
Some Solubility Rules for Common Salts and Bases Soluble salts and bases Symbols Names Exceptions - NO 3 Nitrates None - C2H3O2 Acetates Silver acetate (AgCH 3COO) is only moderately soluble. - (CH 3COO ) Cl -, Chlorides, Silver halides (Ag X), - - X X Br , and I bromides, and mercury(I) halides (Hg 2 2), *lead(II) halides (Pb 2), and mercury(II) iodide (HgI 2), iodides. *Note: PbCl 2 is slightly soluble in cold water and moderately soluble in hot water. 2- SO 4 Sulfates Barium sulfate (BaSO 4), and lead(II) sulfate (PbSO 4). Calcium sulfate (CaSO 4), Mercury(I) sulfate (Hg 2SO 4), and silver sulfate (Ag 2SO 4) are slightly soluble. - The corresponding bisulfates are more soluble. They are: (HSO 4 ) (Bisulfates) calcium bisulfate [Ca(HSO 4)2], mercury(I) bisulfate [Hg 2(HSO 4)2], and silver bisulfate [Ag (HSO 4)2] Na +, Cations of Some uncommon ones*. + K , + sodium, NH 4 potassium, and *Insoluble: Na 4Sb 2O7 , K 2NaCo(NO 2)6 , (NH 4)2NaCo(NO 2)6 , K 2PtCl 6 , and (NH 4)2 ammonium PtCl 6 . Insoluble salts and bases Symbols Names Exceptions 2- 3- 2- CO 3 , PO 4 , SO 3 , and Carbonates, Carbonates, phosphates, sulfites, and silicates of alkali metals 2- + + SiO 4 . such as sodium (Na ) and potassium (K ), and of ammonium phosphates, + (NH 4 ). sulfites, and silicates. Many acid phosphates, such as Mg(H 2PO 4)2 and Ca(H 2PO 4)2. 2- CrO 4 Chromates Na 2CrO 4, K 2CrO 4, (NH 4)2CrO 4, MgCrO 4, CaCrO 4, Al 2(CrO 4)3, NiCrO 4. -

Standard X-Ray Diffraction Powder Patterns NATIONAL BUREAU of STANDARDS
NBS MONOGRAPH 25—SECTION 1 9 CO Q U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard X-ray Diffraction Powder Patterns NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau's technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essentia! services leading to accurate and uniform physical and chemical measurement throughout the Nation's scientific community, industry, and commerce; conducts materials research leading to improved methods of measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials; and provides calibration -

THE MONATOMIC IONS! 1. What Is the Formula for Silver? Ag 2. What Is
Name: ______________________________ THE MONATOMIC IONS! 1. What is the formula for silver? Ag+ 22. What is the formula for cobalt (II)? Co2+ 2. What is the formula for cadmium? Cd2+ 23. What is the formula for chromium (II)? Cr2+ 3. What is the formula for manganese (II)? Mn2+ 24. What is the formula for copper (II)? Cu2+ 4. What is the formula for nickel (II)? Ni2+ 25. What is the formula for tin (IV)? Sn4+ 5. What is the formula for chromous? Cr2+ 26. What is the formula for lead (IV)? Pb4+ 6. What is the formula for zinc? Zn2+ 27. What is the formula for iron (III)? Fe3+ 2+ 2+ 7. What is the formula for cobaltous? Co 28. What is the formula for mercury (I)? Hg2 8. What is the formula for cuprous? Cu+ 29. What is the formula for lead (II)? Pb2+ 9. What is the formula for ferrous? Fe2+ 30. What is the formula for mercury (II)? Hg2+ 2+ 2+ 10. What is the formula for mercurous? Hg2 31. What is the formula for iron (II)? Fe 11. What is the formula for stannous? Sn2+ 32. What is the formula for copper (I)? Cu+ 12. What is the formula for plumbous? Pb2+ 33. What is the formula for tin (II)? Sn2+ 13. What is the formula for chromic? Cr3+ 34. What is the formula for fluoride? F- 14. What is the formula for cobaltic? Co3+ 35. What is the formula for chloride? Cl- 15. What is the formula for cupric? Cu2+ 36. What is the formula for hydride? H- 16. -

Sr( Oh) 2 Soluble Or Insoluble
Sr( oh) 2 soluble or insoluble Continue The question is: Is sr (OH)2 (strontium hydroxy) soluble or insoluble in water? Answer: Sr(OH)2 (Strontium Hydroxide) soluble in water What is soluble and insoluble? Solubility Solubility is a property of a solid, liquid or gas-eating chemical called soluble solvent in solid, liquid or gas-vulnerable solvents. The soluble of the substance depends on the physical and chemical properties of the solvent, as well as on the temperature, pressure and pH of the solution. The degree of solubility of the substance in a particular solvent is measured as the concentration of saturation, in which the addition of the solution does not increase the concentration of the solution and begins to precipitate the excess amount of the solution. The soluble of the substance is completely different from the speed of the solution, which is how quickly it dissolves. Insoluble Term insoluble often applied to bad or very poorly soluble compounds. The overall threshold for describing something as insoluble is less than 0.1 grams per 100 ml of solvent. Soluble List KClO3 ( Potassium chlorate ) KNO3 ( Potassium nitrate ) K2CO3 ( Potassium carbonate ) LiNO3 ( Lithium nitrate ) MgBr2 ( Magnesium bromide ) NaI ( Sodium iodide ) KC2H3O2 ( potassium acetate ) FeSO4 ( Iron(II) sulfate ) CuSO4 ( Copper sulfate ) Na2S ( sodium sulfide ) Na3PO4 ( Trisodium phosphate ) RbCl ( Rubidium chloride ) BaBr2 ( Barium bromide ) AlCl3 ( Aluminium chloride ) HNO3 ( Nitric acid ) FeCl2 ( Iron dichloride ) BaI2 ( Barium iodide ) MnCl2 ( Manganous -

SILVER COMPOUNDS 1. Introduction Silver, a White, Lustrous Metal
SILVER COMPOUNDS 1. Introduction Silver, a white, lustrous metal, slightly less malleable and ductile than gold (see GOLD AND GOLD COMPOUNDS), has high thermal and electrical conductivity (see SIL- VER AND SILVER ALLOYS). Most silver compounds are made from silver nitrate [7761- 88-8], AgNO3, which is prepared from silver metal. Some silver metal is found in Nature, frequently alloyed with other metals such as copper, lead, or gold. Naturally occurring silver compounds, however, are the primary sources of silver in the environment. The most abundant naturally occurring silver compound is silver sulfide [21548- 73-2] (argentite), Ag2S, found alone and combined with iron, copper, and lead sulfides. Other naturally occur- ring silver compounds are silver sulfonantimonite [15983-65-0] (pyrargyrite), Ag3SbS3, silver arsenite [15122-57-3] (proustite), Ag3AsS3, silver selenide [1302-09-6], and silver telluride [12653-91-7]. Silver chloride (chlorargyrite) and silver iodide (iodargyrite) have also been found in substantial quantities in the western United States. Silver is a soft metal that preferentially forms lower oxidation states (þ1, þ2) and binds preferentially with sulfur (1). Studies show that silver levels in uncontaminated fine-grain sediments are typically similar to those for average crustal abundance, 0.05mg/g concentration of silver in fresh lake and river waters is typically<0.2mg/L, while those in contaminated estuary and harbor waters may be upward of from 5.1 to 33.9mg/g (2) produces a linear co-ordination of connecting bonds (3). It has one of the highest electronegativities resulting in strong covalent bonds. Its oxides are weakly held and are dissociate at about 2008C.