Odonata: Lestidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 June 2021 Researchgate: Researchgate.Net/Profile
DAVID OUTOMURO PRIEDE, PH.D. CURRICULUM VITAE June 2021 Researchgate: researchgate.net/profile/David_Outomuro ORCID: orcid.org/0000-0002-1296-7273 EDUCATION Ph.D. 2011 University of Oviedo, Spain (Biology). Summa cum laude. (Dr. Francisco J. Ocharan) B.S. 2005 University of Oviedo, Spain (Biology). Valedictorian. PROFESSIONAL EXPERIENCE Aug 2017- Aug 2021 Postdoctoral researcher, Dept. Biological Sciences, University of Cincinnati, USA (Dr. Nathan Morehouse) Jul 2015-Jun 2017 Postdoctoral researcher, Evolutionary Biology Centre, Uppsala University, Sweden (Drs. Frank Johansson, Anders Ödeen, & Karin Nordström) Jul 2014-Jul 2015 Visiting Professor, Dept. Ciencias Biológicas, Universidad de los Andes, Colombia Nov 2011-Dec 2013 Postdoctoral researcher, Evolutionary Biology Centre, Uppsala University, Sweden (Dr. Frank Johansson) Jun 2006-May 2010 Graduate researcher and Teaching assistant, Dept. Biología de Organismos y Sistemas, University of Oviedo, Spain (Dr. Francisco J. Ocharan) Jul 2005-Aug 2005 Intern, Servicio Regional de Investigación y Desarrollo Agroalimentario de Asturias (SERIDA), Spain (Dr. Isabel Feito Díaz) Sep 2004-Jun 2005 Undergraduate research fellow, Dept. Biología de Organismos y Sistemas, University of Oviedo, Spain (Dr. Francisco J. Ocharan) RESEARCH INTERESTS I am a behavioral ecologist, interested in the micro- and macroevolutionary processes that promote diversity. My research has explored questions on the evolution of color signals, color vision, and flight morphology. I am particularly interested in understanding the evolution of color signals, how they are perceived by intended and unintended receivers and the role of these audiences in driving population and species divergence. I also study the evolution of flight morphology because wings are large conspicuous body surfaces that can be also used as motion signal vehicles for intra- and interspecific communication. -

Dragonflies and Damselflies in Your Garden
Natural England works for people, places and nature to conserve and enhance biodiversity, landscapes and wildlife in rural, urban, coastal and marine areas. Dragonflies and www.naturalengland.org.uk © Natural England 2007 damselflies in your garden ISBN 978-1-84754-015-7 Catalogue code NE21 Written by Caroline Daguet Designed by RR Donnelley Front cover photograph: A male southern hawker dragonfly. This species is the one most commonly seen in gardens. Steve Cham. www.naturalengland.org.uk Dragonflies and damselflies in your garden Dragonflies and damselflies are Modern dragonflies are tiny by amazing insects. They have a long comparison, but are still large and history and modern species are almost spectacular enough to capture the identical to ancestors that flew over attention of anyone walking along a prehistoric forests some 300 million river bank or enjoying a sunny years ago. Some of these ancient afternoon by the garden pond. dragonflies were giants, with This booklet will tell you about the wingspans of up to 70 cm. biology and life-cycles of dragonflies and damselflies, help you to identify some common species, and tell you how you can encourage these insects to visit your garden. Male common blue damselfly. Most damselflies hold their wings against their bodies when at rest. BDS Dragonflies and damselflies belong to Dragonflies the insect order known as Odonata, Dragonflies are usually larger than meaning ‘toothed jaws’. They are often damselflies. They are stronger fliers and referred to collectively as ‘dragonflies’, can often be found well away from but dragonflies and damselflies are two water. When at rest, they hold their distinct groups. -

Bedfordshire and Luton County Wildlife Sites
Bedfordshire and Luton County Wildlife Sites Selection Guidelines VERSION 14 December 2020 BEDFORDSHIRE AND LUTON LOCAL SITES PARTNERSHIP 1 Contents 1. INTRODUCTION ........................................................................................................................................................ 5 2. HISTORY OF THE CWS SYSTEM ......................................................................................................................... 7 3. CURRENT CWS SELECTION PROCESS ................................................................................................................ 8 4. Nature Conservation Review CRITERIA (modified version) ............................................................................. 10 5. GENERAL SUPPLEMENTARY FACTORS ......................................................................................................... 14 6 SITE SELECTION THRESHOLDS........................................................................................................................ 15 BOUNDARIES (all CWS) ............................................................................................................................................ 15 WOODLAND, TREES and HEDGES ........................................................................................................................ 15 TRADITIONAL ORCHARDS AND FRUIT TREES ................................................................................................. 19 ARABLE FIELD MARGINS........................................................................................................................................ -

Bedfordshire Naturalist
The BEDFORDSHIRE NATURALIST BEING THE JOURNAL OF THE BEDFORDSHIRE NATURAL HISTORY SOCIETY AND FIELD CLUB FOR THE YEAR 1958 No. 13 Price Five Shillings PUBLISHED BY THE BEDFORDSHIRE NATURAL HISTORY SOCIETY & FIELD CLUB BEDFORD STONEBRIDGES PRINTERS LIMITED 1959 BEDFORDSHIRE NATURAL HISTORY SOCIETY & FIELD CLUB 1959 President I. G. DONY, Ph.D. Past Presidents SIR FREDERICK MANDER, M.A., B.Sc. OLIVER G. PIKE, F.R.P.S., M.B.O.D. MAJOR SIMON WHITBREAD, B.A., D.L., I.P. Chairman F. G. R. SOPER, I.P. Hon. General Secretary HENRY A. S. KEY, M.P.S., 61B GOLDINGTON ROAD, BEDFORD. Hon. Programme Secretary L. A. SPEED, 226 GOLDINGTON ROAD, BEDFORD. Hon. Treasurer I. M. DYMOND, 91 PUTNOE LANE, BEDFORD. Hon. Editor A. W. GUPPY, 53 GRANGE LANE, BROMHAM. Hon. Librarian MISS E. PROCTOR, B.Sc., THE NATURE ROOM, 4 THE AVENUE, BEDFORD. Council W. DURANT MIssE. PROCTOR A. I. DYMOND- (Co-opted) S. W. RODELL I. I. N. FERGUSON-LEES MISS G. M. TATTAM W. G. HARPER R. G. STEPHENSON E. MEADOWS B. B. WEST K. E. WEST RECORDERS BOTANY: Except Fungi: I. G. Dony, Ph.D., 41 Somerset Avenue, Luton. Fungi: D. A. Reid, B.Sc., The Herbarium, Royal Botanical Gardens, METEOROLOGY: Kew. A. W. -Guppy, B.Sc., .53 Grange Lane, Bromham. PALAEONTOLOGY: P. I., Smart, F.R.E.S., 1 Laburnum Avenue, Bedford. ZOOWGY: Crustacea: Miss E. Proctor, B.Sc., 253 Goldington Road, Bedford. Insecta: Hymenoptera: V. H. Chambers, Ph.D., 47 Westbourne Road, Luton. Lepidoptera: B. B. West, 37 Cardington Road, Bedford. Odonata: K. E. West, 37 Cardington Road, Bedford. -

Libellen Als Indikatoren Für Den Erfolg Von Renaturierungsmaß
© Naturkdl. Station Stadt Linz/Austria; download unter www.biologiezentrum.at BEWERTUNG – ENTOMOLOGIE ÖKO·L 36/2 (2014): 17-26 Univ. Doz. Dr. Andreas Libellen als Indikatoren für den CHOVANEC Erfolg von Renaturierungsmaß- Umweltbundesamt Spittelauer Lände 5 nahmen an Fließgewässern 1090 Wien andreas.chovanec@ am Beispiel der Krems im Bereich umweltbundesamt.at Ansfelden/Oberaudorf Abb. 1: Schematische Darstellung des Untersuchungsgebietes mit den sechs Untersuchungsabschnitten (A-F). Die Anga- ben zur Strömungsgeschwindigkeit in den Abschnitten beziehen sich auf niedrige bis mittlere Wasserführungen; strichlierte Linie: Autobahn A1 Abb. 2: Abschnitt A, regulierter Abschnitt der Krems. Die Verbesserung des ökologischen Zustandes der heimischen Fließgewässer stellt eine der größten zukünftigen Herausfor- derungen für die österreichische Wasserwirtschaft dar. Das Fließgewässer-Netz in Oberösterreich umfasst insgesamt etwa 18.000 km. Von den Gewässern mit einem Einzugsgebiet > 10km2 (Länge 5.400 km) erreichen nur knapp 14 % den sehr guten oder guten ökologischen Zustand (BMLFUW 2010). Es gilt insbesondere, die Durchgängigkeit des Längskontinuums wiederherzustellen, Gewässer mit Umland und Zubringern zu vernetzen sowie die Habitatvielfalt monotoner Flussläufe durch Restrukturierungen zu steigern. In vielen Fällen erfolgen diese Maßnahmen im Zusammenhang mit Projekten zur Erhöhung der Hochwassersicherheit. Die im Jahr 2008 fertiggestellten hydrologischer und morphologischer auf Veränderungen ihres Lebens- Maßnahmen an der unteren Krems Sicht besonderen Vorrang haben. raumes reagieren (z. B. RAAB 2002, im Bereich Ansfelden/Oberaudorf Ziel der vorliegenden Studie war die CHOVANEC u. a. 2012). Libellen sind sind ein Beispiel für wasserbau- Bewertung der an der Krems durch- dem in Wasserrahmenrichtlinie liche Eingriffe, die sowohl den geführten Maßnahmen aus libellen- und Wasserrechtsgesetz (WRRL, ökologischen Zustand als auch den kundlicher Sicht. -

Género Lestes Clave De Especies Ref:Odo.13 (01/03/15)
Género Lestes Clave de especies Ref:Odo.13 (01/03/15) Lestes: Género de los llamados “caballitos del diablo” (Zygoptera, Odonata) que se caracterizan por posarse casi siempre con las alas extendidas. Presentan coloración metálica, a menudo con alguna parte pruinosa azul en los machos y pterostigmas rectangulares notables. En la Península se encuentran citadas seis especies. Lestes barbarus Nota: la presente clave no es válida para individuos recién emergidos, en que la coloración no ha empezado a desarrollarse. A pesar de que las especies se pueden identificar con caracteres macroscópicos bien definidos, el género Lestes Leach, 1815 no es tan sencillo como parece inicialmente y las características descritas deben aplicarse con precaución, así como comprobar los caracteres secundarios que se indican en cada caso para confirmar la identificación. 1.- (1a) Pterostigmas de color pardo claro. Lateral del tórax con una marca distintiva en forma de cuña. Machos con apéndices anales claros.......................................................................................................Lestes viridis (Vander Linden, 1825) Foto 1: Lestes viridis, macho. Se señalan los pterostigmas de color Foto 2: Lestes viridis, macho. Se señalan la marca en forma de cuña pardo pálido (a) y los apéndices anales claros (b). del lateral del tórax (a) y los pterostigmas pardo pálido (b). Foto 3: Lestes viridis, hembra. Se señalan los pterostigmas de color Foto 4: Lestes viridis, hembra. Se señalan la marca en forma de pardo pálido. cuña del lateral del tórax (a) y los pterostigmas pardo pálido. 1 Género Lestes Clave de especies Ref:Odo.13 (01/03/15) (1b) Pterostigmas oscuros o bicolores. Marca en forma de cuña ausente...........................................................................2 2.- (2a) Banda antehumeral reducida o prácticamente inexistente. -

Journal Vol 26 No 2, October 2010
J. Br. Dragonfly Society, Volume 26 No. 2, October 2010 Journal of the CONTENTS DAVID CHELMICK - Studying British dragonflies in the British Dragonfly Society 1970s: the wilderness years .............................................. 57 Volume 26 Number 2 October 2010 BARRY NATTRESS - Folding wing behaviour in Cordulagaster boltonii (Donovan) ............................................................. 64 DAVID CHELMICK - Species Review 4: The Scarce Emerald Damselfly Lestes dryas Kirby with notes on the family Lestidae in the Western Palearctic ....................................66 JONATHAN. R. DIXON & DOROTHY E. GENNARD - The influence of meteorological conditions on the flight activity of the Blue-tailed Damselfly Ischnura elegans (Vander Linden), the Azure Damselfly Coenagrion puella (Linnaeus) and the Emerald Damselfly Lestes sponsa (Hansemann) ..... .............................................................................................. 83 ADRIAN J. PARR -. Migrant and dispersive dragonflies in Britain during 2009 ............................................................97 PAM TAYLOR & DAVE SMALLSHIRE - A change in status of the Dainty Damselfly Coenagrion scitulum (Rambur) in the United Kingdom ………....................................................107 Corrigendum ..........................................................................i The aims of the British Dragonfly Society (BDS) are to promote and encourage the study and conservation INSTRUCTIONS TO AUTHORS of Odonata and their natural habitats, especially in the -

British Dragonfly Society the Scarce Emerald Damselfly Lestes Dryas Kirby 1890
British Dragonfly Society Draft Management Fact File Please note: These MFFs are still draft versions. Any comments sent to the Conservation Officer would be most welcome. The Scarce Emerald Damselfly Lestes dryas Kirby 1890 Identification The Scare Emerald Damselfly (L. dryas) is a medium sized metallic green insect that1, unlike most other damselflies, usually rests with its wings half open2 at an angle of approximately 45deg. Once mature the male and female develop different colouration with the males usually being more noticeable than the females. Adult males have bright blue eyes and develop a powder blue pruinescence on the thorax between the wings and on the segments at the top and bottom of the abdomen (Fig. 1). In comparison, the females tend to be a much duller green and consequently are less easy to see (Fig. 2.). The Scarce Emerald Damselfly can easily be confused with the more common Emerald Damselfly (Lestes sponsa)2,3 especially as they are often found together in the same habitat4. However, the Scarce Emerald Damselfly is more robust than its more common counterpart, and has subtle differences such as brighter blue eyes and less extensive pruinescence2 in the male, and different abdominal markings on segment two in the female. The female Scarce Emerald Damselfly has rectangular paired spots as opposed to the rounded marks seen on the female Emerald Damselfly and the side of its body is also chocolate brown. Close examination of the anal appendages or the ovipositor is required to confirm identification beyond doubt3. Lestes larvae and exuviae can be separated from other damselfly species firstly by the shape of the labium and prementum and banding of the caudal lamellæ3. -
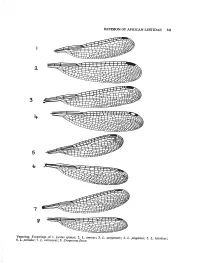
REVISION of AFRICAN LESTIDAE 341 Venation. Forewings of 1. Lestes Sponsa
REVISION OF AFRICAN LESTIDAE 341 Venation. Forewings of 1. Lestes sponsa; 2. L. amicus; 3. L. auripennis; 4. L. plagiatus; 5. L. ictericus; 6. L. palliaus; 7. L, ochraceus; 8. Sytnpecma fusca. 342 ELLIOT PINHEY Wing structures (Text fig. 1-8) Wings usually narrowed apically but apex short, broadly rounded in L. dryas and macro stigma, I. ictericus and some X. ochraceus. TABLE 1. Measurements Shape of mesepisternum Wing characters (normal variation) Start of / Length No. of interc. Abd. Mesepisternum Ratio L/W of Pt Px fore- veins, Subgenus and species ♂ mm Length Width (approx.) mm wing R4-MA fw Lestes sponsa .................. 30 4,1 x 1,3 3 1,25-1,5 11-12 Px 2-3½ L. virens ......................... 26 3,6 x 1,2 3 1,25 9-12 Px 3-5 L. dryas ................. .. 26 4,1 x 1,3 3 1,35-1,5 11-13 Px l½-3 L. barbarus .................. 33 4,8 x 1,3 4 1,25-1,5 10-14 Px 2-4 L. macrostigma.................. 33 3,8 x 1,4 3 2,0-2,5 13-14 Px 3-5 L. virgatus......................... 40 5,4 x 1,3 4 1,5-2 12-14 Px2½-5 L. amicus......................... 37 5,4 X 1,3 4 2,3-3 12-13 Px 4-7 Chalcolestes viridis .. .. 32 3,7 X 1,2 3 1,75 11-13 Px 4-5 Pseudochalcolestes silvaticus 31 4,5 X 1,4 3 1,5-1,75 11-12 Px 3-5 Ps. auripennis .................. 33 4,7 X 1,4 3 2,3-2,5 13-15 Px 4-5 Paralestes tridens . -

Sponsa (Hansemann): Larval
Odonalologica 13 (3): 451-459 September I. 1984 The life history of Lestes sponsa (Hansemann): larval growth (Zygoptera: Lestidae) ¹ J. Pickup,D.J. Thompson¹and J.H. Lawton² ' Department of Zoology, University of Liverpool, P.O. Box 147 Liverpool, L69 3BX, United Kingdom 1 Department of Biology, University of York, Heslington, York, Y0I 5DD, United Kingdom Received March 9, 1983 / Accepted June 25, 1983 The larval L. has growth was monitored in 3 separate populations. sponsa typically 10 instars; larval life lasted from between 68 and 83 days. The sex ratio is close to becomes male but is female biased Females equality initially, biased, at emergence. enter the final instar before males. The population with the fastest growth also showed the greatest degree of synchrony and produced significantly larger larvae than the other populations. It is suggested that food availability accounts for these differences. INTRODUCTION Lestes sponsa (Hans.) typically inhabits acid moorland pools in the British Isles, although it is also found in lowland ponds. It has a wide, Palaearctic distribution(LONGFIELD, 1949). Early workers (e.g. MACAN, 1949, MACNEILL, 1951) supposed that larvae were not found in winter because ofcryptic, mud-dwelling habitsand it was left to CORBET (1956a) to clarify the basic life history of the species, now known to be similar to that of other members of the Lestidae in Europe (e.g. PIERRE, 1904, on L. viridis (Vander L.); WESENBERG-LUND, 1<)13, on L. dryas Kirby), North America (e.g. SAWCHYN & G1LLOTT, 1974a, 1974b, on L. congener Hag., L. unguiculatus Hag., L. disjunctus Sel. and L. -

Atlas of the European Dragonflies and Damselflies Due to Appear During the Past
Atlas of the European dragonflies and damselflies due to appear During the past decade over fifty European odonatologist have been co-operating to bring together all published and unpublished distribution records of the 143 European species of dragonflies and damselflies. The results of this endeavor will appear December 2015 in the Atlas European dragonflies and damselflies (Boudot & Kalkman 2015). The book includes over 200 distribution maps showing both the European and global distribution of the species. Further included is information on taxonomy, range, population trends, flights season-, habitat, photos of nearly all species and for each country an overview of the history of odonatological studies. The book can be pre-ordered for the reduced price of 60 euro’s by sending an e-mail to [email protected] titled 'Special Offer Price Atlas of the dragonflies and damselflies of Europe'. Don't forget to mention your name. You will be contacted when the book is available so that you can order it directly via our webshop. More information on the book, including a preview can be found on http://www.knnvuitgeverij.nl/EN. Vincent Kalkman [email protected] Boudot, J.P. & V.J. Kalkman (eds.) (2015). Atlas of the dragonflies and damselflies of Europe. KNNV- uitgeverij, Netherlands. from agriculture and household sewage (e.g. Belgium), More than 70 % of all European dragonfly species are IUCN Red List categories No. (sub) species Europe (no. endemic species) No. species EU 27 (no. endemic species) and also the construction of dams (e.g. Spain). Outside mentioned in at least one of the national Red Lists. -

Lestes Macrostigma (Eversmann), the Dark Spreadwing
J. Br. Dragonfly Society, Volume 36, No. 2 84 Species Review 11: Lestes macrostigma (Eversmann), the Dark Spreadwing David Chelmick 1 & Philippe Lambret 2 1Macromia Scientific 31 High Beech Lane, Haywards Heath West Sussex UK RH16 1SQ www. macromiascientific.com. 2Tour de Valat, Research Insitute for the Conservation of Mediterranean Wetlands, Le Sambuc, 13200 Arles, France. [email protected] Summary Lestes macrostigma (Dark Spreadwing) occurs across the southern Palearctic from the Atlantic coast as far east as Mongolia. It is locally common in the central part of its range, including Greece and Bulgaria, but becomes increasingly rare in the western parts of the region where, with notable exceptions, it is essentially coastal. It is very much associated with temporary, brackish and saline habitats and, very often, with Bolboschoenus maritimus (Sea Club Rush). Many of its coastal habitats are under threat, especially from wetland transformation schemes of various types and, for that reason, the species is listed as Endangered in the European Red List. Phylogeny The family Lestidae comprises approximately 150 species (Bridges, 1994; Dijkstra et al., 2013). In the western Palearctic, it comprises three genera: • Sympecma – (Winter Damseflies- 2 species) – dull brown patterned spe- cies providing camouflage for the overwintering adults. The only genus in western Europe with this behaviour. • Chalcolestes – (Willow Emeralds- 2 species) – large metallic green dam- selflies without blue pruinosity. These species usually oviposit in green wood above their breeding habitat. • Lestes – (Emerald Damselflies or Spreadwings - 5 species) – medium to large metallic damselflies with blue pruinosity. 85 J. Br. Dragonfly Society, Volume 36, No. 2 The genus Lestes is one of the most widely distributed, with species ranging over all continents (except Antarctica) from the tropics almost up to the Arctic Circle.