MIXED BREEDING SYSTEM and ENTOMOPHILY in Malachra Capitata L. (MALVACEAE)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pollination and Evolution of Plant and Insect Interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket a Dar, Gh
Journal of Pharmacognosy and Phytochemistry 2017; 6(3): 304-311 E-ISSN: 2278-4136 P-ISSN: 2349-8234 Pollination and evolution of plant and insect interaction JPP 2017; 6(3): 304-311 Received: 03-03-2017 Accepted: 04-04-2017 Showket A Dar, Gh. I Hassan, Bilal A Padder, Ab R Wani and Sajad H Showket A Dar Parey Sher-e-Kashmir University of Agricultural Science and Technology, Shalimar, Jammu Abstract and Kashmir-India Flowers exploit insects to achieve pollination; at the same time insects exploit flowers for food. Insects and flowers are a partnership. Each insect group has evolved different sets of mouthparts to exploit the Gh. I Hassan food that flowers provide. From the insects' point of view collecting nectar or pollen is rather like fitting Sher-e-Kashmir University of a key into a lock; the mouthparts of each species can only exploit flowers of a certain size and shape. Agricultural Science and This is why, to support insect diversity in our gardens, we need to plant a diversity of suitable flowers. It Technology, Shalimar, Jammu is definitely not a case of 'one size fits all'. While some insects are generalists and can exploit a wide and Kashmir-India range of flowers, others are specialists and are quite particular in their needs. In flowering plants, pollen grains germinate to form pollen tubes that transport male gametes (sperm cells) to the egg cell in the Bilal A Padder embryo sac during sexual reproduction. Pollen tube biology is complex, presenting parallels with axon Sher-e-Kashmir University of guidance and moving cell systems in animals. -

Toward Understanding the Ecological Impact of Transportation Corridors
United States Department of Agriculture Toward Understanding Forest Service the Ecological Impact of Pacific Northwest Research Station Transportation Corridors General Technical Report PNW-GTR-846 Victoria J. Bennett, Winston P. Smith, and July 2011 Matthew G. Betts D E E P R A U R T LT MENT OF AGRICU The Forest Service of the U.S. Department of Agriculture is dedicated to the principle of multiple use management of the Nation’s forest resources for sustained yields of wood, water, forage, wildlife, and recreation. Through forestry research, cooperation with the States and private forest owners, and management of the National Forests and National Grasslands, it strives—as directed by Congress—to provide increasingly greater service to a growing Nation. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, 1400 Independence Avenue, SW, Washington, DC 20250-9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. Authors Victoria J. -

Pollination in Brazilian Syngonanthus (Eriocaulaceae) Species: Evidence for Entomophily Instead of Anemophily
Annals of Botany 96: 387–397, 2005 doi:10.1093/aob/mci191, available online at www.aob.oupjournals.org Pollination in Brazilian Syngonanthus (Eriocaulaceae) Species: Evidence for Entomophily Instead of Anemophily CARLIANNE O. C. RAMOS*, EDUARDO L. BORBA* and LI´GIA S. FUNCH Departamento de Cieˆncias Biolo´gicas, Laborato´rio de Taxonomia Vegetal, Universidade Estadual de Feira de Santana, Rodovia BR 116, km 03, Feira de Santana, BA, 44031-460, Brazil Received: 17 February 2005 Returned for revision: 1 April 2005 Accepted: 7 May 2005 Published electronically: 20 June 2005 Background and Aims The reproductive biology of Syngonanthus mucugensis and S. curralensis (Eriocaulaceae) was studied in areas of ‘campo rupestre’ vegetation in the Chapada Diamantina, north-eastern Brazil. These species are herbaceous and the individuals have a grouped distribution. Their leaves are united in a rosette, and their inflorescence is monoecious, of the capitulum type. The staminate and pistillate rings mature in a centripetal manner on the capitulum. Methods A field study was conducted, including observations concerning the morphology and biology of the flowers, fruit development, insect visits and anemophily, in both S. mucugensis and S. curralensis. Experimental pollinations were also carried out to study the mating systems of S. mucugensis. Key Results Both species flower from June to August. The staminate cycle lasts approx. 7 d, and the pistillate cycle from 3 to 4 d, with no temporal overlap between them on the same capitulum. The pollen viability of S. mucugensis was 88Á6 %, and 92Á5 % for S. curralensis. The inflorescences of both species demonstrated ultraviolet absorbance, and a sweet odour was detected during both the staminate and pistillate phases. -

Agents for Pollination by Durgeshwer Singh
AGENTS FOR POLLINATION Durgeshwer Singh Department of Botany Mahatma Gandhi Central University Agents for Pollination As the pollen is not capable of locomotion, pollination involves some agents for transfer of pollen grains especially in case of cross pollination. Cross Pollination Abiotic Agents Biotic Agents Entomophily Anemophily Ornithophily Hydrophily Cheiropteriphily Malacophily ABIOTIC AGENTS Anemophily (Pollination by air/ wind) Adaptation • Flowers- inconspicuous, usually not brightly coloured or scented • Petals are either small and green or absent • Male flowers are more numerous than female • Anther are versatile so that they swing freely by air currents • Pollen grains are smooth walled, relatively light, small and dry so they can be easily blown away by wind. • In grasses, pollen grains are relatively heavy and hence are not suitable for transport by wind. To overcome this problem, the male flowers are borne in the upper part of the inflorescence and the female in the lower part. • Examples; Most cereals and palms, Member of Salicaceae (Poplar, willow), Betulaceae (Alder, hazel, birch), Fagaceae (Oak, beech), Ulmaceae (Elm), Urticaceae (Urtica) etc. Hydrophily (Pollination by water) Hydrophilous flower are small and inconspicuous like anemophilous Hypo-hydrophily Epi-hydrophily • Pollination takes place completely under • Pollination of flower at the surface of water. water • More common • Example - Vallisneria • Pollination of flower below water level • Whole male flower break and float on the and is found in submerged plants like surface. Najas, Ceratophyllum and Zostera • Female flower are raised to the surface by • Aerenchyma present in anther- float a long spiral stalk. BIOTIC AGENTS Most important agent for pollination • Entomophily: pollination by Insects • Ornithophily: pollination by birds • Chiropteriphily: pollination by bats • Malacophily: pollination by slug and snail Entomophily (Pollination by insects) • Most frequent in Angiosperms. -

Floral Biology of Myristica Insipida (Myristicaceae), a Distinctive Beetle Pollination Syndrome Author(S): Joseph E
Floral Biology of Myristica insipida (Myristicaceae), a Distinctive Beetle Pollination Syndrome Author(s): Joseph E. Armstrong and Anthony K. Irvine Source: American Journal of Botany, Vol. 76, No. 1 (Jan., 1989), pp. 86-94 Published by: Botanical Society of America, Inc. Stable URL: http://www.jstor.org/stable/2444777 Accessed: 06-09-2016 16:15 UTC REFERENCES Linked references are available on JSTOR for this article: http://www.jstor.org/stable/2444777?seq=1&cid=pdf-reference#references_tab_contents You may need to log in to JSTOR to access the linked references. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at http://about.jstor.org/terms Botanical Society of America, Inc. is collaborating with JSTOR to digitize, preserve and extend access to American Journal of Botany This content downloaded from 132.198.8.49 on Tue, 06 Sep 2016 16:15:01 UTC All use subject to http://about.jstor.org/terms Amer. J. Bot. 76(1): 86-94. 1989. FLORAL BIOLOGY OF MYRISTICA INSIPIDA (MYRISTICACEAE), A DISTINCTIVE BEETLE POLLINATION SYNDROME' JOSEPH E. ARMSTRONG AND ANTHONY K. IRVINE Department of Biological Sciences, Illinois State University, Normal, Illinois 61761; and CSIRO Tropical Forest Research Centre, PO Box 780, Atherton, Queensland 4883, Australia ABSTRACT The floral biology and pollination of Myristica insipida were studied in two different rain forest communities in Queensland. -

A Report on Various Beneficial Roles of Insects
International Journal of Entomology Research International Journal of Entomology Research ISSN: 2455-4758; Impact Factor: RJIF 5.24 Received: 04-11-2019; Accepted: 05-12-2019 www.entomologyjournals.com Volume 5; Issue 1; January 2020; Page No. 15-17 A report on various beneficial roles of insects Deepak Rawal Assistant Professor, Department of Zoology, MLSU Udaipur, Rajasthan, India Abstract It is estimated that out of approximately one million recorded insect species, less than 1000 species are actually pests which transmit diseases and damage crops. They are important part of food chains of many invertebrates, birds, reptiles, amphibians, fishes and even mammals. They are also important in survival of insect pollinated flowering plants. Insects plays variety of roles in nature like pollinators, predators of pests, parasitoids of pests, weed killers, scavengers, decomposers, soil builders, food provider etc. insect are also useful in medicines and have aesthetic as well as scientific values. Currently beneficial insects are widely used in integrative pest management (IPM) and integrative crop management (ICM). Out of million insect species currently we are using only a few insect species for our benefits but I think the scope of insects in welfare of mankind is enormous and we must look in this direction. We must think how we can get more and sustainable benefit from these millions of insect species. Keywords: insects, pollinators, apiculture, sericulture, entomophagy, bioindicators Introduction develops in galls of flowers mates with females inside galls. Most insects are generally considered as pests but it is a Mated wasps then emerges out of flower with lot of pollen wrong notion. -

Insect Ecology & Integrated Pest Management
Insect Ecology & Integrated Pest Management Insect Ecology & Integrated Pest Management This eCourse Developed By TNAU (ICAR) INDEX SN Chapter Page No 1 Principles of Applied Entomology 5-7 2 Honey bees:- History of bee keeping 8-13 3 APIARY MANAGEMENT 14-17 4 ROLE OF HONEY BEES IN CROSS POLLINATION 18-21 5 BEE PRODUCTS - THEIR PROPERTIES AND USES 22-23 6 Effect of agricultural inputs on bee activity – Pesticide poisoning 24-29 7 Role of pollinators, weed killers and other beneficial insects 30-41 8 Insect ecology and balance of life 42-46 9 Population dynamics and role of biotic factors 47-50 10 Abiotic factors on insect population 51-54 11 PEST 55-59 12 PEST MONITORING 60-64 13 PEST MANAGEMENT 65-67 14 TRADITIONAL METHODS OF PEST CONTROL 68-75 15 LEGAL CONTROL METHODS 76-79 16 HOST PLANT RESISTANCE 80-83 17 BIOLOGICAL CONTROL 84-88 18 CHEMICAL CONTROL 89-91 19 PESTICIDES GROUPS 92-94 20 THE INSECTICIDES ACT, 1968 95-97 21 PHEROMONES 98-100 22 STERIITY METHODS 101-103 23 INSECT GROWTH REGULATORS 104-110 24 PESTICIDE APPLICATION METHODS 111-114 25 PESTICIDE COMPATIBILITY 115-120 26 IMPACT OF GLOBAL WARMING ON PESTS 121-122 27 INTEGRATED PEST MANAGEMENT 123-125 28 INTEGRATED PEST MANAGEMENT 126-127 29 IPM (Integrated Pest Management) for Rice 128-129 30 BIOTECHNOLOGY IN PEST MANAGEMENT 130-131 Insect Ecology & Integrated Pest Management Lecture 1 Principles of Applied Entomology The field of entomology may be divided into 2 major aspects. 1. Fundamental Entomology or General Entomology 2. -

Pollination Ecology of an Alpine Fell-Field Community in the North
Oavid C.,Shaw d,o Ronald J. Taylor D.o".tr.a'l,o, Boroq, Wo F W.' r'o .t,.o, L 86 g.d !4o11lo 98ll5 Polljnation Ecologyof an Alpine Fell-FieldCommunity in the North Cascades Abstract 'ruh-!f '{ rlr loliiirlion.''1!1og\of an alpir tul]ficld lo.areit ir LheNIounr Ilater areaoJ washingtonStar. ras ronducred iru.nsth' Lumtrrr''ol l93l llllhation svndrocsGtraregie!)ofth.dorrinanrptantswerearemophrrv.g,ine.arjze.t.nlomoph ilr' and sl"ializ'd ent'nophilv lrs.cr 'Ihe !isitationsro ptantspecies werc q"-tir;,"t "".l i"""",g prenorog! nut $as denrin,n"u. inldtant pollinrrorsrere t,unblcl,rs, srrplid nr"., -a ,,-*"ri iii"., "i'];,". ,",0",,,,,"" $ere hurrerliesand p'imitivf llic' Plantserhitririne specializ*1 entornophily appearcdro oinin;e comperi,i"' l, .""p"-,r"" of ltowerjng NIanyot rheplants lrhiliring rinrcs. gL:neralizedenrooopt,ili norercrt s1mhron",.r,, p".r,.,p.f* ' "r" .,\ ,,",a*,arion to alra.t h.ge nunbcrs 1. 1, ,..r.,i,.. 1 , r-t'^\ .,.,4Lhi F tt,{pr: Introduction rcducrng competition for pollinators. Foraging behavior of flies and butterflies is variable Pollin.rriuner-ul'rgr i. a rapidh gror.ing -uh- anJ "olJ-fa.lrr,,rrnJ' lesslell known (Proctor and yco l9?3, Faegri dr- inline.l bi,,log\.rccen \ e,ndVand"r Pijl l,r7q,.In gnrreral.ili,.. proier erpanJirrglrnrrr .r nrinrarilrdc", riprir, to an .i- llo\1crj sith n,rn-con.erl,d rerarrl., Jo not perimenlal science.Howerer, although therc are .peeializ".und appcarirr rlpirr,.r"ommunitie. a, number of excellent tcxts, c.g., Faegri and earll in the season.Buttcrflies preler tubular Vander Pijl (1979),Jones and LitJe (1983),proc- llorcrs with toncealed rcwards, have a higher tor and Yeo (1973), and Richards (1978), ancl level of floral constancythan flies, and app"ar nunrerou:arlr"ter , "nc, rningpollination biolugr. -
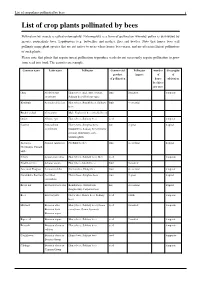
List of Crop Plants Pollinated by Bees 1 List of Crop Plants Pollinated by Bees
List of crop plants pollinated by bees 1 List of crop plants pollinated by bees Pollination by insects is called entomophily. Entomophily is a form of pollination whereby pollen is distributed by insects, particularly bees, Lepidoptera (e.g. butterflies and moths), flies and beetles. Note that honey bees will pollinate many plant species that are not native to areas where honey bees occur, and are often inefficient pollinators of such plants. Please note that plants that require insect pollination to produce seeds do not necessarily require pollination to grow from seed into food. The carrot is an example. Common name Latin name Pollinator Commercial Pollinator number Geography product impact of of of pollination honey cultivation bee hives per acre Okra Abelmoschus Honey bees (incl. Apis cerana), fruit 2-modest temperate esculentus Solitary bees (Halictus spp.) Kiwifruit Actinidia deliciosa Honey bees, Bumblebees, Solitary fruit 4-essential bees Bucket orchid Coryanthes Male Euglossini bees (Orchid bees) Onion Allium cepa Honey bees, Solitary bees seed temperate Cashew Anacardium Honey bees, Stingless bees, nut 3-great tropical occidentale bumblebees, Solitary bees (Centris tarsata), Butterflies, flies, hummingbirds Atemoya, Annona squamosa Nitidulid beetles fruit 4-essential tropical Cherimoya, Custard apple Celery Apium graveolens Honey bees, Solitary bees, flies seed temperate Strawberry tree Arbutus unedo Honey bees, bumblebees fruit 2-modest American Pawpaw Asimina triloba Carrion flies, Dung flies fruit 4-essential temperate Carambola, -

Pollination Biology
Pollination Biology . real story of the birds & bees . and beetles, bugs, butterflies, bats Sexual Reproduction in Plants • Movement onto land is an issue for sexual reproduction in plants - unlike for animals • rely on movement of (1) pollen, (2) young embryo encased in a seed (or fruit), or (3) spores pollination biology seed dispersal Sexual Reproduction in Plants Pollination and seed/spore dispersal important aspects of biosystematics in plants: • Gene flow • Outcrossing vs. inbreeding • Reproductive isolation • Speciation spore dispersal • Co-speciation (coevolution) pollination biology seed dispersal Coevolution Coevolution – interactions between two different clades as selective forces on each other, resulting in adaptations that increase their interdependency Animal-flowering plant interaction is a classic example of coevolution: • Plants evolve elaborate methods to attract animal pollinators • Animals evolve specialized body parts and behaviors that aid plant pollination ! Coevolution109 • coevolution with pollinators often leads to convergence and divergence in flowers • best studied has been the phlox family: Polemoniaceae Fig. 1. Floral diversity in Polemoniaceae. (A) Leptosiphon aureus subsp. aureus; (B) Saltugilia splendens subsp. grantii; (C) Navarretia hamata subsp. hamata; (D) Leptosiphon montanus; (E) Phlox divaricata subsp. laphamii; (F) Cantua buxifolia; (G) Aliciellla latifolia subsp. latifolia; (H) Linanthus orcutii; (I) Saltugilia caruifolia; (J) Loeseliastrum schottii; (K) Cobaea scandens; (L) Eriastrum eremicum -

Biodiversity – Bee Week a Curriculum and Activity Guide for Bee Awareness Middle School Curriculum
Biodiversity – Bee Week A curriculum and activity guide for Bee Awareness Middle School Curriculum Encyclopedia of Life / BioLib.cz / © Ivo Antusek Mary Klass, Geological Society of America, Geocorps America Program; Retired Poudre School District, Fort Collins, CO, Science Teacher Sally Plumb, National Park Service, Natural Resources Stewardship and Science May 1, 2015 Biodiversity—Bee Week Middle School Curriculum I. Introduction II. Curriculum Outline A. Day 1 Topic: External Observation of a Honey Bee Focus Question: What adaptations do bees have to be effective pollinators and to survive? Warm-Up: Students will watch short video “Flight of the Bumble (Honey) Bee” to introduce different bee adaptations. As the video is being shown, the instructor will point out different structures that students are going to see in their external observation lab of the honey bee. Lesson: External Observation of a Honey Bee: Students will follow detailed directions on a lab sheet to complete an external observation of a honey bee, observing the different adaptations that help the bees be effective pollinators and survive. Post Lesson Assessment: Think – Pair – Share: The instructor will pose question to whole class: “What is the most important adaptation that a bee has to be an effective pollinator and to survive? Students will “Think – Pair – Share” their ideas. B. Day 2 Topic: Flower Dissection / Insect Pollination Focus Question: What adaptations do flowers have to ensure their pollination and survival? Warm-Up: Flowers for the lab should be displayed in a central area where students can look at them. The instructor will write this statement on board for students to answer: Give five examples of how the flowers are different and five examples of how the flowers are alike. -

Department of Plant Protection
DEPARTMENT OF PLANT PROTECTION M.Sc. Ag. (Entomology) CBCS II Semester Core Course- IV: INSECT ECOLOGY LECTURE NO. 1 INSECT ECOLOGY The word ecology is the modified form of ‘Oekologie’ derived from the Greek ‘Oikos’, meaning ‘Home’ and ‘Logos’ meaning ‘Discourse’ introduced by Reiter in 1869 and later anglicized to ‘Ecology’. Ecology is a multidisciplinary subject and derives support from other sciences. Individual organisms of the same species live together as a ‘Population’. Population can be defined as ‘a group of individuals or a species occurring in a given area or locality at a specific time’. Populations of different species live together and form a ‘Community’, meaning ‘all populations in the area at a specific time’. The community is influenced by its physical environment. The complex system of biotic and abiotic factors constitutes an ‘Ecosystem’. Whereas the crops, insects, other animals and the physical abiotic factors together constitute an ‘Agro-ecosystem’. Ecology is ‘the science of inter-relations between living organisms and their environment including both the physical and the biotic environments and emphasizing inter species and intra species relations’ (Allee, 1949). Odum (1953) defined ecology as ‘the study of the structure and functions of nature (or Environmental biology)’. Ecology is divided mainly into ‘Autecology’ and ‘Synecology’. Autecology is the study of individual organisms or an individual species in relation to the environment while Synecology is the study of the group or groups of organisms associated in a community in the same environment i.e., in relation to various other species living in the same environment. Importance of Ecology in Pest Management: Indiscriminate uses of pesticides lead to a regular resurgence of pests due to the fact that the natural enemies get killed.