Semi-Empirical Software for the Aluminothermic and Carbothermic Reactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preparation of Alumina-Chromium Composites by Reactive Hot-Pressing A1 + Cr2o3 Based Powders D
Preparation of alumina-chromium composites by reactive hot-pressing A1 + Cr2O3 based powders D. Osso, A. Mocellin, Gérard Le Caër, A. Pianelli To cite this version: D. Osso, A. Mocellin, Gérard Le Caër, A. Pianelli. Preparation of alumina-chromium composites by reactive hot-pressing A1 + Cr2O3 based powders. Journal de Physique IV Proceedings, EDP Sciences, 1993, 03 (C7), pp.C7-1311-C7-1316. 10.1051/jp4:19937202. jpa-00251836 HAL Id: jpa-00251836 https://hal.archives-ouvertes.fr/jpa-00251836 Submitted on 1 Jan 1993 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE IV Colloque C7, supplement au Journal de Physique 111, Volume 3, novembre 1993 Preparation of alumina-chromium composites by reactive hot-pressing A1 + Cr203 based powders D. OSSO, A. MOCELLIN, G. LE CAER and A. PIANELLI LSG2M, URA I59 du CNRS, Ecole des Mines, 54042 Nancy cedex, France Chromium-Alumina based composites have been obtained by reactive sintering under load and vacuum of various powder blends. The starting mixtures have been prepared from commercially available aluminium metal, chromium and aluminium oxides, and a thermally unstable titanium compound respectively. Differential thermal analysis (DTA) and differential calorimetry (DSC) as well as X-ray diffraction were used to identify chemical transformations taking place within the system. -

Chromium 28.07.2020.Pdf
Chromium Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard and brittle transition metal. Chromium is the main additive in stainless steel, to which it adds anti-corrosive properties. Chromium is also highly valued as a metal that is able to be highly polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, with almost 90% of infrared light being reflected. Ferrochromium alloy is commercially produced from chromite by silicothermic or aluminothermic reactions and chromium metal by roasting and leaching processes followed by reduction with carbon and then aluminium. Chromium metal is of high value for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Ores:- Although chromium occurs in many minerals, the only ore exploited commercially is chromite. This spinel mineral is ideally composed of ferrous oxide and chromic oxide with the chemical composition FeO · Cr2O3, but it is often found in nature with magnesia (MgO) substituting for FeO and alumina (A12O3) or ferric oxide (Fe2O3) substituting for Cr2O3. Other minerals such as silica (SiO2) are also present. Extraction:- Chromite, FeCr2O4, is the most commercially useful ore, and is extensively used for extraction of chromium. Chromium is produced in two forms: (a) Ferrochrome by the reduction of chromite with coke in an electric arc furnace. -

Synthesis of Li2s-Carbon Cathode Materials Via Carbothermic Reduction of Li2so4
ORIGINAL RESEARCH published: 04 June 2019 doi: 10.3389/fenrg.2019.00053 Synthesis of Li2S-Carbon Cathode Materials via Carbothermic Reduction of Li2SO4 Jiayan Shi 1, Jian Zhang 2, Yifan Zhao 2, Zheng Yan 1, Noam Hart 1 and Juchen Guo 1,2* 1 Department of Chemical and Environmental Engineering, University of California, Riverside, Riverside, CA, United States, 2 Materials Science and Engineering Program, University of California, Riverside, Riverside, CA, United States Pre-lithiated sulfur materials are promising cathode for lithium-sulfur batteries. The synthesis of lithium sulfide-carbon (Li2S-C) composite by carbothermic reduction of lithium sulfate (Li2SO4) is investigated in this study. The relationship between reaction temperature and the consumption of carbon in the carbothermic reduction of Li2SO4 is first investigated to precisely control the carbon content in the resultant Li2S-C composites. To understand the relationship between the material structure and the electrochemical properties, Li2S-C composites with the same carbon content are subsequently synthesized by controlling the mass ratio of Li2SO4/carbon and the reaction temperature. Systematic electrochemical analyses and microscopic characterizations Edited by: demonstrate that the size of the Li S particles dispersed in the carbon matrix is the key Wen Liu, 2 Beijing University of Chemical parameter determining the electrochemical performance. A reversible capacity of 600 Technology, China −1 mAh g is achieved under lean electrolyte condition with high Li2S areal loading. -

The Preparation of Chromium Metal by a Sealed, Cold-Hearth, Plasma-Assisted Aluminothermic Method
The preparation of chromium metal by a sealed, cold-hearth, plasma-assisted aluminothermic method by L.R. Nelson * applications of aluminothermic-reduction technology to the production of metals. Synopsis Future expansion of aluminothermic metal production depends, in part, on the capability The efficiency and cost-effectiveness of the aluminothennic to prove that metal oxides from new sources reduction process in the production of ferro-alloys and 'pure' are amenable to processing. For evaluation of metals depends strongly on appropriate balancing of the process the suitability to aluminothermic reduction of energy required. Insufficient energy results in a poor metal yield, chromic oxide from a unique source, a sealed, while over-supply can result in excessive fuming and possibly even cold-hearth, plasma-assisted test unit was ejection of the reactor contents in a potentially dangerous 'thennite developed. This provided a means of evaluating bomb' reaction. This situation becomes even more critical when the suitability of limited quantities of oxide from new sources must be the aluminothermic preparation of 'pure' evaluated, dictating smaller-scale testwork. The resulting higher chromium metal from a chromic oxide source. relative heat losses from the system complicates control of the This paper gives details of the technique and energy balance, and usually requires the addition of thermal describes the experimental results. boosters or reactant preheating in order to sustain an autothermic process. This often detracts from the quality of the metal product, Process energy required and adds to the hazards of batch aluminothennic-reduction testing. The development of a small-scale method (producing less than General aluminothermic processes 2,5 kg of metal) for the plasma-assisted aluminothennic reduction of chromium oxide that overcomes such difficulties is described. -

Manufacturer Thermite Weld (Pakistan) EST
THERMITE WELDING NEW GENERATION WELDING Manufacturer Thermite Weld (Pakistan) EST. Office: Room No. 1, 3rd Floor Raja Chamber, 35-Queens Road, Lahore. Factory: Shadiwal, Moterway, Thokar Niaz Baig, Lahore 0322-4101587, [email protected], [email protected] www.thermiteweld.com thermiteweld THERMITE WELDING EXECOTHERMICWELD NEW GENERATION WELDING Manufacturer Thermite Weld (Pakistan) EST. Office: Room No. 1, 3rd Floor Raja Chamber, 35-Queens Road, Lahore. Factory: Shadiwal, Moterway, Thokar Niaz Baig, Lahore 0322-4101587, [email protected], [email protected] www.thermiteweld.com thermiteweld THERMITE WELDING EXECOTHERMICWELD NEW GENERATION WELDING Manufacturer Thermite Weld (Pakistan) EST. Office: Room No. 1, 3rd Floor Raja Chamber, 35-Queens Road, Lahore. Factory: Shadiwal, Moterway, Thokar Niaz Baig, Lahore 0322-4101587, [email protected], [email protected] www.thermiteweld.com thermiteweld INTRODUCTION The thermite welding system is a miracle of chemistry an easy simple to use in field process for welding copper to copper, copper to steel, without the use of external source. Thermite connections utilize the high temperature reaction of powdered copper oxide and aluminum, which when ignited, produced aluminum oxide (in the form of slag) and super-heated copper. The reaction takes place oxide with molten metal aluminum in various welding applications. The reaction takes place in a graphite crucible. The pieces to be welded are introduced prior to the reaction. After the powder is ignited, molten metal from the aluminothermic reaction flows around the pieces, causing them to melt and fuse into a solid homogeneous piece. When the total mass of powder becomes supper heated molten copper, it flows through the mould into the conductor to be joined by melting a thin steel disc which previously has stopped the powder from dropping through the mould. -

Carbothermic Reduction Kinetics of Ilmenite Concentrates Catalyzed by Sodium Silicate and Microwave- Absorbing Characteristics O
Available on line at Association of the Chemical Engineers of Serbia AChE www.ache.org.rs/CICEQ Chemical Industry & Chemical Engineering Quarterly 19 (3) 423−433 (2013) CI&CEQ WEI LI1,2 CARBOTHERMIC REDUCTION KINETICS OF JINHUI PENG1 1 ILMENITE CONCENTRATES CATALYZED BY SHENGHUI GUO SODIUM SILICATE AND MICROWAVE- LIBO ZHANG1 GUO CHEN1 ABSORBING CHARACTERISTICS OF HONGYING XIA1 REDUCTIVE PRODUCTS 1 Key Laboratory of Unconventional Carbothermic reduction kinetics of ilmenite concentrates catalyzed by sodium Metallurgy, Ministry of Education, silicate were investigated; the reduction degree of ilmenite concentrates Kunming University of Science and reduction reaction was determined as R = 4/7(16y + 56x)(ΔWΣ - f W)/(16y + Technology, Yunnan, China A-P 2Faculty of Science, Kunming + 56x + 112). The results show that the reaction activation energy of initial University of Science and stage and later stage is 36.45 and 135.14 kJ/mol, respectively. There is a great Technology, Yunnan, China change in the reduction rate at temperatures of 1100 and 1150 °C; the catal- ysis effect and change of reduction rate were evaluated by TG and DSC curves SCIENTIFIC PAPER of sodium silicate. Microwave-absorbing characteristics of reduction products were measured by the method of microwave cavity perturbation. It was found UDC 544.47/.478 that microwave absorbing characteristics of reduction products obtained at ° DOI 10.2298/CICEQ120421077L temperatures of 900, 1100 and 1150 C have significant differences. XRD characterization results explained the formation and accumulation of reduction product Fe, and pronounced changes of microwave absorbing characteristics due to the decrease of the content of ilmenite concentrates. Keywords: sodium silicate; ilmenite concentrates; catalytic reduction; kinetics; microwave absorbing characteristics. -

Manufacturer of Exothermic Weld Powder & Graphite
MANUFACTURER OF EXOTHERMIC WELD POWDER & GRAPHITE MOULDS Exothermic welding, also known as exothermic bonding and is a welding process for joining two electrical conductors, that employs superheated copper alloy to permanently join the conductors. The process employs an exothermic reaction of a copper thermite composition to heat the copper, and requires no external source of heat or current. The chemical reaction that produces the heat is an aluminothermic reaction between aluminum powder and a metal oxide. The reaction reaches very high temperatures, depending on the metal oxide used. The reactants are usually supplied in the form of powders, with the reaction triggered using a spark from a flint lighter. The activation energy for this reaction is very high however, and initiation requires either the use of a “booster” material such as powdered magnesium metal or a very hot flame source. The aluminum oxide slag that it produces is discarded. When welding copper conductors, the process employs a semi- permanent graphite crucible mould, in which the molten copper, produced by the reaction, flows through the mould and over and around the conductors to be welded, forming an electrically conductive weld between them. When the copper cools, the mould is either broken off or left in place. Alternatively, hand-held graphite crucibles can be used. The advantages of these crucibles include portability, lower cost (because they can be reused), and flexibility, especially in field applications. The weld formed has higher mechanical strength than other forms of weld, and excellent corrosion resistance. It is also highly stable when subject to repeated short-circuit pulses, and does not suffer from increased electrical resistance over the lifetime of the installation. -

CARBOTHERMIC REDUCTION of MECHANICALLY ACTIVATED Nio-CARBON MIXTURE: NON-ISOTHERMAL KINETICS
J. Min. Metall. Sect. B-Metall. 54 (3) B (2018) 313 - 322 Journal of Mining and Metallurgy, Section B: Metallurgy CARBOTHERMIC REDUCTION OF MECHANICALLY ACTIVATED NiO-CARBON MIXTURE: NON-ISOTHERMAL KINETICS S. Bakhshandeh, N. Setoudeh *, M. Ali Askari Zamani, A. Mohassel Yasouj University, Materials Engineering Department, Yasouj, Iran (Received 23 March 2018; accepted 10 December 2018) Abstract The effect of mechanical activation on the carbothermic reduction of nickel oxide was investigated. Mixtures of nickel oxide and activated carbon (99% carbon) were milled for different periods of time in a planetary ball mill. The unmilled mixture and milled samples were subjected to thermogravimetric analysis (TGA) under an argon atmosphere and their solid products of the reduction reaction were studied using XRD experiments. TGA showed that the reduction of NiO started at ~800 and ~720 in un-milled and one-hour milled samples respectively whilst after 25h of milling it decreased to about 430 . The kinetics parameters of carbothermic reduction were determined using non-isothermal method (Coats-Redfern Method) for un-milled and milled samples. The activation energy was determined to be about 222 kJ mol -1 for un-milled mixture whilst it was decreased to about 148 kJ mol -1 in 25-h milled sample. The decrease in the particle size/crystallite size of the℃ milled samp℃les resulted in a significant drop in the reaction temperature. ℃ Keywords : Ball milling; Carbothermic reduction; Nickel oxide; Non-isothermal kinetics; Thermo-gravimetric analysis (TGA). 1. Introduction solid product catalyzes the reaction rate [ 11 ]. It has been shown that mechanical activation by There have been many investigations on the milling reactants together with extended periods of carbothermic reduction of metal oxide in the literature time can intensify the reactions [ 16-19 ]. -

Aluminothermic Reduction of Anatase Concentrates Lino
ALUMINOTHERMIC REDUCTION OF ANATASE CONCENTRATES LINO R. FREITAS*, Metallurgical Engineer, M.Sc. and Ph.D. PAULO R. MAZONI*, Metallurgical Engineer MAGID W. SAAB*, Metallurgical Engineer, M.Sc. *Center of Technology, Cornpanhia Vale do Rio Doce, Belo Horizonte-MG, Brazil INFACON 86 PROCEEDINGS 349 ABSTRACT The aluminothermic reduction of anatase concentrates (80-86% Ti0 2 i, from Tapira-MG, Brazil, has been studied in an open, alumina-lined reactor. Thermodynamic calculations, as well as the preliminary experiments, indicated that the reaction is not sufficiently exothermic to enable separation of the products. Therefore, additional heat is necessary and a heat booster such as sodium chlorate (NaC10 3 ) was employed. It was found that NaC10 3 additions in excess of 15% of the Ti0 2 content in the feed charge were required to obtain good metal/slag separation. Batch tests with a maximum charge of 15 kg of concentrate were performed and titanium recoveries up to 74% were obtained. The effect of the following variables on the titanium yield was determined: amount of feed charge, amount of fluxes (lime and fluorspar), particle size of reagents and amount of aluminium in excess of the stoichiometric quantity. Additional tests with Australian rutile and synthetic titanium dioxide as feed materials were performed, with similar results. Also, experiments without boosters, but with external heating were conducted and comparable titanium recoveries were obtained. 350 ALUMINOTHERMIC REDUCTION OF ANATASE INTRODUCTION The aluminothermic reduction, also known as production of titanium by aluminothermic the thermite process, is widely used in the reduction, however, is very limited and preparation of ferro-alloys. -

Carbothermic Production of Hexagonal Boron Nitride A
CARBOTHERMIC PRODUCTION OF HEXAGONAL BORON NITRIDE A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY HASAN ERDEM ÇAMURLU IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN METALLURGICAL AND MATERIALS ENGINEERING NOVEMBER 2006 Approval of the Graduate School of Natural and Applied Sciences Prof. Dr. Canan Özgen Director I certify that this thesis satisfies all the requirements as a thesis for the degree of Doctor of Philosophy. Prof. Dr. Tayfur Öztürk Head of Department This is to certify that we have read this thesis and that in our opinion it is fully adequate, in scope and quality, as a thesis and for the degree of Doctor of Philosophy. Prof. Dr. Yavuz A. Topkaya Prof. Dr. Naci Sevinç Co-Supervisor Supervisor Examining Committee Members Prof. Dr Ahmet Geveci (METU, METE) Prof. Dr. Naci Sevinç (METU, METE) Prof. Dr. Yavuz A. Topkaya (METU, METE) Prof. Dr. Önder Özbelge (METU, CHE) Assoc. Prof. Dr. Nuri Durlu (ETÜ, ME) I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name: Hasan Erdem Çamurlu Signature : ABSTRACT CARBOTHERMIC PRODUCTION OF HEXAGONAL BORON NITRIDE Çamurlu, Hasan Erdem Ph.D., Department of Metallurgical and Materials Engineering Supervisor : Prof. Dr. Naci Sevinç Co-Supervisor: Prof. Dr. Yavuz A. Topkaya October 2006, 124 pages Formation of hexagonal boron nitride (h-BN) by carbothermic reduction of B2O3 under nitrogen atmosphere at 1500oC was investigated. -
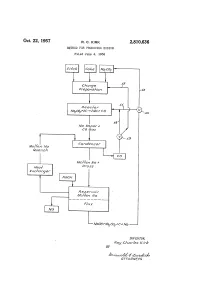
Wooh+Wacof C. Ma
Oct. 22, 1957 R. C. KRK 2,810,636 METHOD FOR PRODUCING SODIUM Filed June 4, 1956 Charge tAeac for Waco, 7-2C-2Wa CO Wa Vooor 7 CO Gas Mo/fern Wa Gornoeraser Guerch Mo/fer? Wa 7. Aea/ O/ro SS Af xcharger Aeser/or Mo/fer? Wa WoOh+WaCOf c. Ma INVENTOR. - Aoy CAar/es kirA BY A77OAAWEYS 2,810,636 United States Patent Office Patented Oct. 22, 1957 1. 2 Some dross is inevitably formed during this condensa tion operation and is separated from the molten sodium 2,810,636 by fluxing with sodium hydroxide. It has been discovered that the carbon monoxide which METHOD FOR PRODUCING SODUM is obtained as a by-product from the reaction of carbon with sodium carbonate will react with the sodium hy Roy.Chemical Charles Company,Kirk, Midland, Midland, Michassignor Mich., a corporation to The Dow of droxide in the flux at temperatures of at least 318 C. Delaware (at which temperature the sodium hydroxide is fluid or molten) to produce sodium carbonate. The thus treated Application June 4, 1956, Serial No. 589,198 0 flux may then be fed into the reactor with added carbon 7 Claims. (C. 75-66) to produce more sodium, since the higher melting sodium carbonate does not attack the graphite lining of the re actor. In this manner a cyclical process is obtained which recovers almost 100 percent of the sodium values present. This invention relates to a process for producing So 5 Since the sodium values in the dross are almost entirely dium and is particularly directed to an improved cyclical recovered, economical modifications of the sodium car method of making sodium by the carbothermic reduction bonate reduction process which previously could not be of sodium carbonate. -

Carbothermic Reduction of Alumina with Carbon in Vacuum
J. Cent. South Univ. (2012) 19: 1813−1816 DOI: 10.1007/s1177101212130 Carbothermic reduction of alumina with carbon in vacuum YU Qingchun(郁青春), YUAN Haibin(袁海滨), ZHU Fulong(朱富龙), ZHANG Han(张晗), WANG Chen(王辰), LIU Dachun(刘大春), YANG Bin(杨斌) National Engineering Laboratory of Vacuum Metallurgy, Key Laboratory of Breeding Base of Complex Nonferrous Metal Resources Clear Utilization in Yunnan Province, State Key Laboratory of Nonferrous Metal Vacuum Metallurgy, Kunming University of Science and Technology, Kunming 650093, China © Central South University Press and SpringerVerlag Berlin Heidelberg 2012 Abstract: Carbothermic reduction alumina in vacuum was conducted, and the products were analysed by means of XRD and gas chromatography. Thermodynamic analysis shows that in vacuum the initial carbothermic reduction reaction temperature reduces compared with that under normal pressure, and the preferential order of products is Al4O4C, Al4C3, Al2OC, Al2O and Al. Experiment results show that the carbothermic reduction products of alumina are Al4O4C and Al4C3, and neither Al2OC, Al2O or Al was found. During the carbothermic reduction process, the reaction rate of Al2O3 and carbon decreases gradually with increasing time. Meanwhile, lower system pressure or higher temperature is beneficial to the carbothermic reduction of alumina process. Al4O4C is firstly formed in the carbothermic reaction, and then Al4C3 is formed in lower system pressure or at higher temperature. Key words: alumina; carbothermic reduction; vacuum; aluminum the formation of aluminum carbide and oxycarbide 1 Introduction byproducts complicates the carbothermic reduction and chlorination process. The thermodynamic study of Aluminum is currently produced industrially via the chemical reactions provides a basic understanding of the HallHe´roult process by dissolving Al2O3 in fused process prior to designing suitable reaction experiments, NaFAlF3 (cryolite) followed by direct current and provides a useful guideline for the selection of electrolysis.