Supporting Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Victor Chow 周 Lock Lee 李 Sun Johnson 孫 Dr on Lee 李 Phillip
Victor Chow 周 Gilbert Yet Ting Quoy 丁 Ben Hing 卿 Lock Lee 李 Yip Ho Nung 葉 Chang Gar Lock 鄭 Sun Johnson 孫 Jimmy Young 楊 Ye Bing Nan 葉 Dr On Lee 李 George Soo Hoo Ten 司徒 Ye Tong Gui 葉 Phillip Lee Chun 李 Chen Ah Teak 陳 Liang Chuang 梁 Mei Quong Tart 梅 Chung Gon, Joseph 鐘 Li Chun 李 W.R.G. Lee 李 Tse Tsan Tai 謝 Xian Jun Hao 冼 Yu Rong 余 Ping Nam 平 Henry Fine-Chong 鄭 Louie Hoon 雷 Bing Guin Lee 李 Ng Hung-pui 伍 Joe Wah Gow 周 James See 時 Wu E Lou 伍 George Gay 蓋 Ah Toy 蔡 Mei Dong Xing 梅 Way Kee 魏 Chang, Victor Peter 鐘 Wong On 王 Lok Kwok 郭 Leon, Lester 梁 Huang Zhu 黃 Chuen Kwon 關 Yong Yew 雍 Ma Ying Biao 馬 Yat Kwan (Ken Wong) 王 Zeng Pinging Chang Woo Gow Thomas Pan Kee 潘 (Percival Chong) 鐘 (Zhan Shichai) 詹 Chee Dock Nomchong 莊 Zeng Yinan Li Bu 李 Shoon Foon Nomchong 莊 (Frederick Chong) 鐘 Yu Xing 余 Ah’ Yung 翁 Thomas Yee Hing 劉 Wan Chang 鄭 Mak Sai Ying Laun Chong 鐘 Zhu Bing Hing 朱 (John Shying) 麥 Lo King Nam 康 Wu Chao Quan 吳 Harry Chinn Kow You Man 寇 Simpson Lee 李 (Tung Chow) 陳 Robert Lee Young 楊 Ouyang Nan 歐陽 Nam You 尤 Hong On Jang 康 Lee Hwa 李 Fong Bing 方 Jas Wong Quong 王 Sun Kum Loong 孫 Liu Guangfu 劉 William Robert George Lee Chen Gong 陳 Kwong Sue Duk 鄺 (Lee Yihui/Yikfai) 李 William Young 楊 John Samuel Yu 余 Dr George On Lee 李 Lee Yuan Xin 李 Olive Mabel James Ung Quoy Huang Lai Wang Clarice Wong 王 (Jang Quoy) 郭 (Samuel Wong) 黃 Wong Ah Sat 王 Gwok Ah Poo 郭 Charlie Hoy 許 Yeesang Loong 龍 Guo Shun 郭 Ah Chuney 秦 William Chiu 邱 Tin Lee 田 Onyik Lee 李 Kwok Bew 郭 Charlie Leanfore Ahchong Wong 王 William Joseph Lumb Liu 劉 (Chan Lean Fore) 詹 Ah Fung 馮 John Young Wai 周 John Juansing zhang 張 Choy Yuen Gum 蔡 陈秋林: 百家姓 Chen Qiulin: One Hundred Names January 16 - February 27, 2016 Artist Bio Insofar as the French Revolution is the Event of modern history, In the following year, whilst undertaking research in Chengdu, the break after which ‘nothing was the same’, one should raise here Sichuan Province experienced one of the most destructive Chen Qiulin (b. -

Appeal No. 2100138 DOB V. Chen Xue Huang March 17, 2021
Appeal No. 2100138 DOB v. Chen Xue Huang March 17, 2021 APPEAL DECISION The appeal of Respondent, premises owner, is denied. Respondent appeals from a recommended decision by Hearing Officer J. Handlin (Bklyn.), dated December 3, 2020, sustaining a Class 1 charge of § 28-207.2.2 of the Administrative Code of the City of New York (Code), for continuing work while on notice of a stop work order. Having fully reviewed the record, the Board finds that the hearing officer’s decision is supported by the law and a preponderance of the evidence. Therefore, the Board finds as follows: Summons Law Charged Hearing Determination Appeal Determination Penalty 35488852J Code § 28-207.2.2 In Violation Affirmed – In Violation $5,000 In the summons, the issuing officer (IO) affirmed observing on July 20, 2020, at 32-14 106th Street, Queens: [T]wo workers at cellar level working on boiler while on notice of stop work order #052318C0303JW.” At the telephonic hearing, held on December 1, 2020, the attorney for Petitioner, Department of Buildings (DOB), relied on the IO’s affirmed statement in the summons and also the Complaint Overview for the premises, which documented Petitioner’s issuance of a full SWO on June 28, 2019, still in effect on the date of inspection. Respondent’s representative stated as follows. Respondent knew about the full SWO; however, Petitioner issued it in conjunction with a job that Respondent withdrew. Respondent then filed a new job that superseded the previous application and for which DOB issued a work permit. In the decision sustaining the charge, the hearing officer found that because Respondent offered no evidence that he ever applied to Petitioner to rescind the SWO, the work observed by the IO resulted in the cited violation. -

The Significance and Inheritance of Huang Di Culture
ISSN 1799-2591 Theory and Practice in Language Studies, Vol. 8, No. 12, pp. 1698-1703, December 2018 DOI: http://dx.doi.org/10.17507/tpls.0812.17 The Significance and Inheritance of Huang Di Culture Donghui Chen Henan Academy of Social Sciences, Zhengzhou, Henan, China Abstract—Huang Di culture is an important source of Chinese culture. It is not mechanical, still and solidified but melting, extensible, creative, pioneering and vigorous. It is the root of Chinese culture and a cultural system that keeps pace with the times. Its influence is enduring and universal. It has rich connotations including the “Root” Culture, the “Harmony” Culture, the “Golden Mean” Culture, the “Governance” Culture. All these have a great significance for the times and the realization of the Great Chinese Dream, therefore, it is necessary to combine the inheritance of Huang Di culture with its innovation, constantly absorb the fresh blood of the times with a confident, open and creative attitude, give Huang Di culture a rich connotation of the times, tap the factors in Huang Di culture that fit the development of the modern times to advance the progress of the country and society, and make Huang Di culture still full of vitality in the contemporary era. Index Terms—Huang Di, Huang Di culture, Chinese culture I. INTRODUCTION Huang Di, being considered the ancestor of all Han Chinese in Chinese mythology, is a legendary emperor and cultural hero. His victory in the war against Emperor Chi You is viewed as the establishment of the Han Chinese nationality. He has made great many accomplishments in agriculture, medicine, arithmetic, calendar, Chinese characters and arts, among which, his invention of the principles of Traditional Chinese medicine, Huang Di Nei Jing, has been seen as one of the greatest contributions to Chinese medicine. -

Digesting Anomalies: an Investment Approach
Digesting Anomalies: An Investment Approach Kewei Hou The Ohio State University and China Academy of Financial Research Chen Xue University of Cincinnati Lu Zhang The Ohio State University and National Bureau of Economic Research An empirical q-factor model consisting of the market factor, a size factor, an investment factor, and a profitability factor largely summarizes the cross section of average stock returns. A comprehensive examination of nearly 80 anomalies reveals that about one-half of the anomalies are insignificant in the broad cross section. More importantly, with a few exceptions, the q-factor model’s performance is at least comparable to, and in many cases better than that of the Fama-French (1993) 3-factor model and the Carhart (1997) 4-factor model in capturing the remaining significant anomalies. (JEL G12, G14) In a highly influential article, Fama and French (1996) show that, except for momentum, their 3-factor model, which consists of the market factor, a factor based on market equity (small-minus-big, SMB), and a factor based on book- to-market equity (high-minus-low, HML), summarizes the cross section of average stock returns as of the mid-1990s. Over the past 2 decades, however, it has become clear that the Fama-French model fails to account for a wide array of asset pricing anomalies.1 We thank Roger Loh, René Stulz, Mike Weisbach, Ingrid Werner, Jialin Yu, and other seminar participants at the 2013 China International Conference in Finance and the Ohio State University for helpful comments. Geert Bekaert (the editor) and three anonymous referees deserve special thanks. -

September 10, 1959 Memorandum of Conversation Between Chinese Vice Foreign Minister Zeng Yongquan and Charge D’Affaires of the Embassy of the Soviet Union in China S
Digital Archive digitalarchive.wilsoncenter.org International History Declassified September 10, 1959 Memorandum of Conversation Between Chinese Vice Foreign Minister Zeng Yongquan and Charge d’Affaires of the Embassy of the Soviet Union in China S. Antonov Citation: “Memorandum of Conversation Between Chinese Vice Foreign Minister Zeng Yongquan and Charge d’Affaires of the Embassy of the Soviet Union in China S. Antonov,” September 10, 1959, History and Public Policy Program Digital Archive, PRC FMA 109-00873-12, 76-79. Obtained by Dai Chaowu and translated by 7Brands. http://digitalarchive.wilsoncenter.org/document/114757 Summary: Zeng and Antonov talked about the outbreak of conflict along the Sino-Indian border, a TASS statement regarding the issue made without incorporating Chinese opinion and the role of publicity. Credits: This document was made possible with support from the MacArthur Foundation. Original Language: Chinese Contents: English Translation Memorandum of Conversation between the Vice Minister [Zeng Yongquan] and Charge d’Affaires of the Embassy of the Soviet Union in China [S.] Antonov Date: 10 September 1959, 12:00 a.m. Place: Ministry of Foreign Affairs Participants: Secretary Li Huichuan and First Secretary of the Embassy of the Soviet Union in China [F.V.] Mochulskii Interpreted and recorded by: Wang [Illegible] S. Antonov: I made an appointment to meet with you yesterday because I met Mr. Zhang Hanfu. I have notified him of the Laotian issue. I did not come to visit you because the issue was already settled. Zeng Yongquan: I know. I came here so late tonight because I have something urgent to tell you. -

The Yu of Yi-Mei and Chang-Wan in Kwangtung,S Xin-Hui Xian
Genealogy and History: The Yu of Yi-mei and Chang-wan in Kwangtung,s Xin-hui xian By L . E ve A r m e n t r o u t M a Chinese / Chinese American History Project, El Cerrito CAt USA A number of reasons have been advanced to explain why formal clan and lineage1 organizations first became widespread in China during the Song dynasty. Among these is the suggestion that the “ new ’, men (many of obscure origins), brought into the bureaucracy by the Song emphasis on the しonfucian examination system, desired to strength en the fabric of society. One means they chose was encouraging the revitalization and extension of clan and lineage organizations. They did tms in order to fill the void created by the decline of the aristocratic (“ great”) families (Twitchett 1959: 100-101). If these assumptions are true, one would expect to find a certain amount of the “ ordinary ” in the genealogies kept by these groups. 1 his was, indeed, often the case. A good example is that of the Yu 余 lineage that I will introduce in this essay. These Yus lived in the villages of Yi-mei 乂美 and Chang-wan 長湾 in Kwangtung’s Xin-hui xian 新会縣. As we shall see, they provide an interesting case study of a lineage that had its brief moment of glory, constructed a genealogy that helped to reflect this moment, and then faded into relative obscurity. In light of what we know about the distribution of wealth and power in the southern Chinese countryside, there must have been thousands of lineages similar to this one. -

Social Mobility in China, 1645-2012: a Surname Study Yu (Max) Hao and Gregory Clark, University of California, Davis [email protected], [email protected] 11/6/2012
Social Mobility in China, 1645-2012: A Surname Study Yu (Max) Hao and Gregory Clark, University of California, Davis [email protected], [email protected] 11/6/2012 The dragon begets dragon, the phoenix begets phoenix, and the son of the rat digs holes in the ground (traditional saying). This paper estimates the rate of intergenerational social mobility in Late Imperial, Republican and Communist China by examining the changing social status of originally elite surnames over time. It finds much lower rates of mobility in all eras than previous studies have suggested, though there is some increase in mobility in the Republican and Communist eras. But even in the Communist era social mobility rates are much lower than are conventionally estimated for China, Scandinavia, the UK or USA. These findings are consistent with the hypotheses of Campbell and Lee (2011) of the importance of kin networks in the intergenerational transmission of status. But we argue more likely it reflects mainly a systematic tendency of standard mobility studies to overestimate rates of social mobility. This paper estimates intergenerational social mobility rates in China across three eras: the Late Imperial Era, 1644-1911, the Republican Era, 1912-49 and the Communist Era, 1949-2012. Was the economic stagnation of the late Qing era associated with low intergenerational mobility rates? Did the short lived Republic achieve greater social mobility after the demise of the centuries long Imperial exam system, and the creation of modern Westernized education? The exam system was abolished in 1905, just before the advent of the Republic. Exam titles brought high status, but taking the traditional exams required huge investment in a form of “human capital” that was unsuitable to modern growth (Yuchtman 2010). -

English Versions of Chinese Authors' Names in Biomedical Journals
Dialogue English Versions of Chinese Authors’ Names in Biomedical Journals: Observations and Recommendations The English language is widely used inter- In English transliteration, two-syllable Forms of Chinese Authors’ Names nationally for academic purposes. Most of given names sometimes are spelled as two in Biomedical Journals the world’s leading life-science journals are words (Jian Hua), sometimes as one word We recently reviewed forms of Chinese published in English. A growing number (Jianhua), and sometimes hyphenated authors’ names accompanying English- of Chinese biomedical journals publish (Jian-Hua). language articles or abstracts in various abstracts or full papers in this language. Occasionally Chinese surnames are Chinese and Western biomedical journals. We have studied how Chinese authors’ two syllables (for example, Ou-Yang, Mu- We found considerable inconsistency even names are presented in English in bio- Rong, Si-Ma, and Si-Tu). Editors who are within the same journal or issue. The forms medical journals. There is considerable relatively unfamiliar with Chinese names were in the following categories: inconsistency. This inconsistency causes may mistake these compound surnames for • Surname in all capital letters followed by confusion, for example, in distinguishing given names. hyphenated or closed-up given name, for surnames from given names and thus cit- China has 56 ethnic groups. Names example, ing names properly in reference lists. of minority group members can differ KE Zhi-Yong (Chinese Journal of In the current article we begin by pre- considerably from those of Hans, who Contemporary Pediatrics) senting as background some features of constitute most of the Chinese population. GUO Liang-Qian (Chinese Chinese names. -
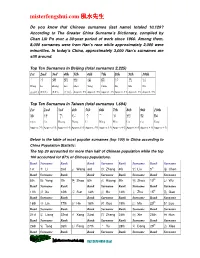
Misterfengshui.Com 風水先生
misterfengshui.com 風水先生 Do you know that Chinese surnames (last name) totaled 10,129? According to The Greater China Surname’s Dictionary, compiled by Chan Lik Pu over a 30-year period of work since 1960. Among them, 8,000 surnames were from Han’s race while approximately 2,000 were minorities. In today’s China, approximately 3,000 Han’s surnames are still around. Top Ten Surnames In Beijing (total surnames 2,225) 1st 2nd 3rd 4th 5th 6th 7th 8th 9th 10th 王 李 張 劉 趙 楊 陳 徐 馬 吳 Wang Li Zhang Liu Zhao Yang Chen Xu Ma Wu (10.6% ) (9.6%) (9.6%) (7.7% ) Approx. 5% Approx. 5% Approx. 4% Approx. 4% Approx. 4% Approx. 3% Top Ten Surnames In Taiwan (total surnames 1,694) 1st 2nd 3rd 4th 5th 6th 7th 8th 9th 10th 陳 林 黃 張 李 王 吳 劉 蔡 楊 Chen Lin Huang Zhang Li Wang Wux. Liu Cai Yang Approx .7% Approx 6 % Approx 6 % Approx 6 % Approx. 5% Approx 5 % Approx 4 % Approx 4 % Approx 4 % Approx 4 % Below is the table of most popular surnames (top 100) in China according to China Population Statistic: The top 20 accounted for more than half of Chinese population while the top 100 accounted for 87% of Chinese populations. Rank Surname Rank Rank Surname Rank Surname Rank Surname 1st 李 Li 2nd 王 Wang 3rd 張 Zhang 4th 劉 Liu 5th 陳 Chen Rank Surname Rank Rank Surname Rank Surname Rank Surname 6th 楊 Yang 7th 趙 Zhao 8th 黃 Huang 9th 周 Zhou 10 th 吳 Wu Rank Surname Rank Rank Surname Rank Surname Rank Surname 11th 徐 Xu 12th 孫 Sun 13th 胡 Hu 14th 朱 Zhu 15 th 高 Gao Rank Surname Rank Rank Surname Rank Surname Rank Surname 16th 林 Lin 17th 何 He 18th 郭 Guo 19th 馬 Ma 20 th 羅 Luo Rank -

Representing Talented Women in Eighteenth-Century Chinese Painting: Thirteen Female Disciples Seeking Instruction at the Lake Pavilion
REPRESENTING TALENTED WOMEN IN EIGHTEENTH-CENTURY CHINESE PAINTING: THIRTEEN FEMALE DISCIPLES SEEKING INSTRUCTION AT THE LAKE PAVILION By Copyright 2016 Janet C. Chen Submitted to the graduate degree program in Art History and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ________________________________ Chairperson Marsha Haufler ________________________________ Amy McNair ________________________________ Sherry Fowler ________________________________ Jungsil Jenny Lee ________________________________ Keith McMahon Date Defended: May 13, 2016 The Dissertation Committee for Janet C. Chen certifies that this is the approved version of the following dissertation: REPRESENTING TALENTED WOMEN IN EIGHTEENTH-CENTURY CHINESE PAINTING: THIRTEEN FEMALE DISCIPLES SEEKING INSTRUCTION AT THE LAKE PAVILION ________________________________ Chairperson Marsha Haufler Date approved: May 13, 2016 ii Abstract As the first comprehensive art-historical study of the Qing poet Yuan Mei (1716–97) and the female intellectuals in his circle, this dissertation examines the depictions of these women in an eighteenth-century handscroll, Thirteen Female Disciples Seeking Instructions at the Lake Pavilion, related paintings, and the accompanying inscriptions. Created when an increasing number of women turned to the scholarly arts, in particular painting and poetry, these paintings documented the more receptive attitude of literati toward talented women and their support in the social and artistic lives of female intellectuals. These pictures show the women cultivating themselves through literati activities and poetic meditation in nature or gardens, common tropes in portraits of male scholars. The predominantly male patrons, painters, and colophon authors all took part in the formation of the women’s public identities as poets and artists; the first two determined the visual representations, and the third, through writings, confirmed and elaborated on the designated identities. -

Names of Chinese People in Singapore
101 Lodz Papers in Pragmatics 7.1 (2011): 101-133 DOI: 10.2478/v10016-011-0005-6 Lee Cher Leng Department of Chinese Studies, National University of Singapore ETHNOGRAPHY OF SINGAPORE CHINESE NAMES: RACE, RELIGION, AND REPRESENTATION Abstract Singapore Chinese is part of the Chinese Diaspora.This research shows how Singapore Chinese names reflect the Chinese naming tradition of surnames and generation names, as well as Straits Chinese influence. The names also reflect the beliefs and religion of Singapore Chinese. More significantly, a change of identity and representation is reflected in the names of earlier settlers and Singapore Chinese today. This paper aims to show the general naming traditions of Chinese in Singapore as well as a change in ideology and trends due to globalization. Keywords Singapore, Chinese, names, identity, beliefs, globalization. 1. Introduction When parents choose a name for a child, the name necessarily reflects their thoughts and aspirations with regards to the child. These thoughts and aspirations are shaped by the historical, social, cultural or spiritual setting of the time and place they are living in whether or not they are aware of them. Thus, the study of names is an important window through which one could view how these parents prefer their children to be perceived by society at large, according to the identities, roles, values, hierarchies or expectations constructed within a social space. Goodenough explains this culturally driven context of names and naming practices: Department of Chinese Studies, National University of Singapore The Shaw Foundation Building, Block AS7, Level 5 5 Arts Link, Singapore 117570 e-mail: [email protected] 102 Lee Cher Leng Ethnography of Singapore Chinese Names: Race, Religion, and Representation Different naming and address customs necessarily select different things about the self for communication and consequent emphasis. -

Zhenzhong Zeng School of Environmental Science And
Zhenzhong Zeng School of Environmental Science and Engineering, Southern University of Science and Technology [email protected] | https://scholar.google.com/citations?user=GsM4YKQAAAAJ&hl=en CURRENT POSITION Associate Professor, Southern University of Science and Technology, since 2019.09.04 PROFESSIONAL EXPERIENCE Postdoctoral Research Associate, Princeton University, 2016-2019 (Mentor: Dr. Eric F. Wood) EDUCATION Ph.D. in Physical Geography, Peking University, 2011-2016 (Supervisor: Dr. Shilong Piao) B.S. in Geography Science, Sun Yat-Sen University, 2007-2011 RESEARCH INTERESTS Global change and the earth system Biosphere-atmosphere interactions Terrestrial hydrology Water and agriculture resources Climate change and its impacts, with a focus on China and the tropics AWARDS & HONORS 2019 Shenzhen High Level Professionals – National Leading Talents 2018 “China Agricultural Science Major Progress” - Impacts of climate warming on global crop yields and feedbacks of vegetation growth change on global climate, Chinese Academy of Agricultural Sciences 2016 Outstanding Graduate of Peking University 2013 – 2016 Presidential (Peking University) Scholarship; 2014 – 2015 Peking University PhD Projects Award; 2013 – 2014 Peking University "Innovation Award"; 2013 – 2014 National Scholarship; 2012 – 2013 Guanghua Scholarship; 2011 – 2012 National Scholarship; 2011 – 2012 Founder Scholarship 2011 Outstanding Graduate of Sun Yat-Sen University 2009 National Undergraduate Mathematical Contest in Modeling (Provincial Award); 2009 – 2010 National Scholarship; 2008 – 2009 National Scholarship; 2007 – 2008 Guanghua Scholarship PUBLICATION LIST 1. Liu, X., Huang, Y., Xu, X., Li, X., Li, X.*, Ciais, P., Lin, P., Gong, K., Ziegler, A. D., Chen, A., Gong, P., Chen, J., Hu, G., Chen, Y., Wang, S., Wu, Q., Huang, K., Estes, L. & Zeng, Z.* High spatiotemporal resolution mapping of global urban change from 1985 to 2015.