Wild Orangutan Tool Use NEW INSIGHTS for HUMAN EVOLUTION
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bornean Orangutans
Envisioning a future for BORNEAN ORANGUTANS Conservation impacts of 10 years of Bornean action plan implementation and recommendations for improved population outcomes Authors: Julie Sherman, Marc Ancrenaz, and Erik Meijaard Protected areas, forest change and forest fragment maps and analysis by Maria Voigt cover illustration : Lucy Molleson CONTENTS Evaluating Bornean orangutan conservation effectiveness ........................................................................ 1 Results ................................................................................................................................................................... 2 Plan implementation ................................................................................................................................. 2 Stakeholder interventions .......................................................................................................................... 3 Rescue and release .................................................................................................................................... 4 Law enforcement ....................................................................................................................................... 6 Management of orangutans in concessions ............................................................................................... 6 Habitat loss and habitat protection ........................................................................................................... 7 Orangutan -

Orangutan SAFE Program Plan
2020-2022 Orangutan SAFE Program Plan ORANGUTAN SAFE PROGR AM PLAN Contents Orangutan SAFE Species Program Goal ............................................................................................................................. 2 Program Operational Structure ............................................................................................................................................ 2 Conservation Target ................................................................................................................................................................ 3 Status of Species within the AZA Community ................................................................................................................... 3 AZA Conservation Activities ................................................................................................................................................. 4 AZA Public Engagement Activities ...................................................................................................................................... 5 Conservation Status of the Species ...................................................................................................................................... 5 Population and Habitat Viability Assessment ..................................................................................................................... 6 Threats .................................................................................................................................................................................... -

The Population and Distribution of Orangutans (Pongo Pygmaeus Pygmaeus) in and Around the Danau Sentarum Wildlife Reserve, West Kalimantan, Indonesia
Biological Conservation 97 (2001) 21±28 www.elsevier.com/locate/biocon The population and distribution of orangutans (Pongo pygmaeus pygmaeus) in and around the Danau Sentarum Wildlife Reserve, West Kalimantan, Indonesia Anne E. Russon a,*, Andi Erman b, Rona Dennis c aPsychology Department, Glendon College, 2275 Bayview Avenue, Toronto, ON, Canada M4N 3M6 bWetlands International Indonesia, Danau Sentarum Wildlife Reserve, West Kalimantan, Indonesia cODA-MOFEC Indonesia±UK Tropical Forest Management Project, Bogor, Indonesia Received 29 September 1998; received in revised form 9 February 2000; accepted 13 May 2000 Abstract We report estimates of orangutan distribution and population size in and around the Danau Sentarum Wildlife Reserve (DSWR) in West Kalimantan, Indonesia, one of the few areas in Borneo that may still support a large population. We generated estimates in line with each of three proposed de®nitions for the reserve Ð its current boundaries, a moderate extension, and a greater extension. Estimates were based on current nest survey methods, adjusted for habitat type variability. Measures of habitat type coverage derived from a GIS developed for the DSWR region. Our estimates support impressions that the current DSWR harbors few orangutans (Æ200) but the greater extension supports a substantially larger population (>2000). Findings are discussed with respect to distribution (habitat preference), behavior in the DSWR area (migration, local stresses, human tolerance), and habitat protection plans. # 2000 Elsevier Science Ltd. All rights reserved. Keywords: Orangutans; Borneo; Orangutan distribution; Population estimation; Protection plans 1. Introduction agricultural and commercial use. The most notorious of these, which aimed to convert a million hectares of good The IUCN classi®es orangutans (Pongo pygmaeus)as orangutan habitat to agriculture (Presidential Decree vulnerable, i.e. -
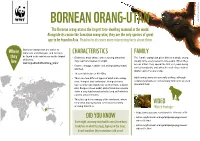
ORANG-UTAN the Bornean Orang-Utan Is the Largest Tree-Dwelling Mammal in the World
© naturepl.com / Anup Shah / WWF © naturepl.com / BORNEAN ORANG-UTAN The Bornean orang-utan is the largest tree-dwelling mammal in the world. Alongside its cousin the Sumatran orang-utan, they are the only species of great ape to be found in Asia. Read on to discover more interesting facts about them. Bornean orang-utans are native to Where Indonesia and Malaysia, and can only CHARACTERISTICS FAMILY be found in the rainforests on the Island they • Distinctive body shape, with very long arms that The female orang-utan gives birth to a single young of Borneo. live may reach 2.2 metres in length. usually once every seven to nine years. When they wwf.org.uk/wildlife/orang_utan/ are an infant, they spend the first 2-3 years being • Coarse, shaggy, reddish coat and grasping hands carried constantly, and will still remain close to their and feet. mother up to 7 years of age. • They weigh between 40-85kg. • There are two different types of adult male orang- Adult orang-utans are generally solitary, although utan: ‘flanged’ and ‘unflanged’. Flanged males temporary groups are occasionally formed in areas of have a long coat of dark hair on their back, a facial abundant food. disk, flanges (cheek pads) and a throat sac used to make a very loud sound called a ‘long call’ which is used to attract females. • They live up in the canopy of the rainforest, where they climb and swing and even build a nest by VIDEO weaving branches. Borneo Watch footage: • https://www.youtube.com/watch?v=ft2ntmJTm7Y DID YOU KNOW • arkive.org/bornean-orangutan/pongo-pygmaeus/ Each night, an orang-utan builds a nest from bent video-00.html branches on which to sleep, high up in the trees. -

9 Orangutan Tool Use and the Evolution of Technology
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2013 Orangutan tool use and the evolution of technology Meulman, E J M ; van Schaik, C P DOI: https://doi.org/10.1017/CBO9780511894800.011 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-92534 Book Section Published Version Originally published at: Meulman, E J M; van Schaik, C P (2013). Orangutan tool use and the evolution of technology. In: Sanz, C M; Call, J; Boesch, C. Tool Use in Animals. Cognition and Ecology. Cambridge, UK: Cambridge University Press, 176-202. DOI: https://doi.org/10.1017/CBO9780511894800.011 C:/ITOOLS/WMS/CUP-NEW/3473718/WORKINGFOLDER/SANZ/9781107011199C09.3D 176 [176--202] 19.10.2012 11:24AM 9 Orangutan tool use and the evolution of technology E. J. M. Meulman Anthropological Institute and Museum, University of Zurich C. P. van Schaik Anthropological Institute and Museum, University of Zurich Introduction Commonly referred to as a hallmark of human evolution, tool use is often considered a complex skill. Paradoxically, however, tool use seems to be widespread in the animal kingdom and may consist of fairly simple behavioral actions. In this chapter we try to relate these somewhat contradictory views to the relatively rare occurrence of habitual and complex tool use in wild orangutans, especially when compared to wild chimpanzees. We propose that, in addition to the previously suggested factors (i.e., extractive foraging, social tolerance and intelligence), terrestriality may have been instrumental in the evolution of especially habitual (sensu McGrew & Marchant, 1997) and complex tool use, thus explaining the “orangutan tool paradox.” Our preliminary comparison of eight orangutan and ten chimpanzee study populations (descriptively, via a principal component analysis (PCA), and by testing predictions related to the four factors) does indeed point in this direction. -

Traffic Southeast Asia Report
HANGING IN THE BALANCE: AN ASSESSMENT OF TRADE IN ORANG-UTANS AND GIBBONS ON KALIMANTAN,INDONESIA VINCENT NIJMAN A TRAFFIC SOUTHEAST ASIA REPORT TRAFFIC SOUTHEAST ASIA Published by TRAFFIC Southeast Asia, Petaling Jaya, Selangor, Malaysia © 2005 TRAFFIC Southeast Asia All rights reserved. All material appearing in this publication is copyrighted and may be produced with permission. Any reproduction in full or in part of this publication must credit TRAFFIC Southeast Asia as the copyright owner. The views of the authors expressed in this publication do not necessarily reflect those of the TRAFFIC Network, WWF or IUCN. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organizations concerning the legal status of any country, territory, or area, or its authorities, or concerning the delimitation of its frontiers or boundaries. The TRAFFIC symbol copyright and Registered Trademark ownership is held by WWF, TRAFFIC is a joint programme of WWF and IUCN. Layout by Noorainie Awang Anak, TRAFFIC Southeast Asia Suggested citation: Vincent Nijman (2005), Hanging in the Balance: An Assessment of trade in Orang-utans and Gibbons in Kalimantan, Indonesia TRAFFIC Southeast Asia ISBN 983-3393-03-9 Photograph credit: Pet Müller’s Gibbon Hylobates muelleri, West Kalimantan, Indonesia (Ian M. Hilman/Yayasan Titian) Hanging in the Balance: An Assessment of Trade in Orang-utans and Gibbons in Kalimantan, Indonesia HANGING IN THE BALANCE: An assessment of trade in orang-utans and gibbons in Kalimantan, Indonesia Vincent Nijman August 2005 Yuyun Kurniawan/Yayasan Titian Kurniawan/Yayasan Yuyun Credit: Credit: Orang-utan and macaque skulls used for decoration in Central Kalimantan. -

Wild Bornean Orangutans Experience Muscle Catabolism During Episodes of Fruit Scarcity Caitlin A
www.nature.com/scientificreports OPEN Wild Bornean orangutans experience muscle catabolism during episodes of fruit scarcity Caitlin A. O’Connell1*, Andrea L. DiGiorgio2,1, Alexa D. Ugarte1,3, Rebecca S. A. Brittain1, Daniel J. Naumenko4,5, Sri Suci Utami Atmoko6,7 & Erin R. Vogel1 Pronounced temporal and spatial variation in the availability of food resources can produce energetic defcits in organisms. Fruit-dependent Bornean orangutans face extreme variation in fruit availability and experience negative energy and protein balance during episodes of fruit scarcity. We evaluate the possibility that orangutans of diferent sexes and ages catabolize muscle tissue when the availability of fruit is low. We assess variation in muscle mass by examining the relationship between urinary creatinine and specifc gravity and use the residuals as a non-invasive measure of estimated lean body mass (ELBM). Despite orangutans having a suite of adaptations to bufer them from fruit scarcity and associated caloric defcits, ELBM was lower during low fruit periods in all age-sex classes. As predicted, adult male orangutans had higher ELBM than adult females and immatures. Contrary to expectation, fanged and unfanged males did not difer signifcantly in ELBM. These fndings highlight the precarity of orangutan health in the face of rapid environmental change and add to a growing body of evidence that orangutans are characterized by unique metabolic traits shaped by their unpredictable forest environment. Te rainforests of Southeast Asia are characterized as challenging habitats for vertebrate frugivores 1. In particu- lar, these rainforests experience greater variation in annual fruit productivity than African and South American rainforests2. Te island of Borneo is particularly limited, with lower overall fruit productivity compared to Sumatra3,4, and this low resource abundance has shaped the island’s fauna in various ways. -

The Great Apes – the Road Ahead. a GLOBIO Perspective on the Impacts
<*** (#) C») [GHMEE # life1 unep A r e n d a 1 GreuUnbniaiPnM UNEP WCMC The Great Apes - the road ahead A Gipbio perspective on the impacts of infrastructural development on The Great Apes Front cover photos Left to right: Daniel O'Brien/UNEP/Topham; Born Free Foundation/I Redmond; G Brower/UNEP/Topham; Born Free Foundation/I Redmond; Tan Yik Yee/UNEP/Topham. Disclaimer The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organizations. The designations employed and the presentations do not imply the expressions of any opinion whatsoever on the part of UNEP or contributory organizations concerning the legal status of any country, territory, city or area or its authority, or concerning the delimitation of its frontiers or boundaries. ^l-?f < /ii*\ f~ > GRID ($') UfJ vt^m io.^ j? unep A r e n d a 1 basttaivmntrma UNEP WCMC The Great Apes - the road ahead A Gipbio perspective on the impacts of infrastructural development on The Great Apes The Great Apes - the road ahead Editors Dr. Christian Nellemann Dr. Adrian Newton Global coordinator, GLOBIO UNEP World Conservation Monitoring Centre UNEPGRID-Arendal 219 Huntingdon Road, Cambridge CB3 ODL, UK c/o Norwegian Institute for Nature Research Tel. +44(0)1223 277314 Fakkelgarden, Storhove Fax +44 (0)1223 277136 N-2624 Lillehammer, Norway e-mail adnan.new1onf3unep-wcmc.org Tel. + 47 612 87900 website http://www.unep-wcmc.org/ Fax + 47 612 87901 e-mail christian.nellemannianina.no website http://www.globio.info/ Contributors Hugo Ahlenius Dr. Ruth de Fries UNEPGRID-Arendal (Remote sensing data for forest cover] Longum Park Department of Geography and Earth System Service Box 706, N-4808 Arendal, Norway Science Interdisciplinary Center e-mail ahleniusiagrida.no University of Maryland website http://www.gnda.no/ College Park Massachusetts, MD 20742, USA Jared Bakuza, Mary Edwards, Dr. -

Great Ape Conservation Fund
U.S. Fish & Wildlife Service Great Ape Conservation Fund Apes, by their has become exacerbated as roads built Additionally, recent outbreaks of nature, are for logging and mining operations hemorrhagic fevers, like Ebola, have extremely allow poachers access to previously reduced some ape populations to a vulnerable. They remote forests, which once served as fraction of their previous numbers, have very low refuges for apes. In villages where killing up to 90% of individuals in densities, grow meat from domesticated animals is affected populations. relatively slowly, scarce and expensive, wild animals are are long-lived, have hunted as a cheap and locally available Apes are susceptible to many of the low reproductive rates, and complex source of protein. In cities, eating same diseases as humans. As growing social relationships. Today, the entire bushmeat has become a status symbol human populations penetrate further world’s great ape species are for the wealthy and the demand for into ape habitat, the potential for threatened with extinction. wild meat has steadily increased. disease transmission between apes and people, and vice versa, increases. As In Asia, both orangutans and gibbons a result, the bushmeat trade poses a are facing a multitude of serious serious health risk for humans. threats. Asia’s only great ape species, the Sumatran orangutan and In response to these many pressing the Bornean orangutan, are found threats, the United States Congress respectively on the islands of Sumatra enacted the Great Ape Conservation and Borneo. They will both be extinct Act in 2000 which created the Great if illegal logging, forest fires, poorly Ape Conservation Fund for the planned road construction, conversion conservation of gorillas, chimpanzees, of indigenous forest to plantations, bonobos, orangutans, and gibbons. -

Drivers of Bornean Orangutan Distribution Across a Multiple-Use Tropical Landscape
remote sensing Article Drivers of Bornean Orangutan Distribution across a Multiple-Use Tropical Landscape Sol Milne 1,*, Julien G. A. Martin 1,2 , Glen Reynolds 3, Charles S. Vairappan 4 , Eleanor M. Slade 5 , Jedediah F. Brodie 6, Serge A. Wich 7, Nicola Williamson 1 and David F. R. P. Burslem 1 1 School of Biological Sciences, University of Aberdeen, Cruickshank Building, St Machar Drive, Aberdeen AB24 3UU, UK; [email protected] (J.G.A.M.); [email protected] (N.W.); [email protected] (D.F.R.P.B.) 2 Department of Biology, University of Ottawa, Gendron Hall, 30 Marie Curie, Ottawa, ON K1N 6N5, Canada 3 South East Asia Rainforest Research Partnership, Danum Valley Field Centre, P.O. Box 60282, Lahad Datu 91112, Sabah, Malaysia; [email protected] 4 Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Jalan UMS, Kota Kinabalu 88400, Sabah, Malaysia; [email protected] 5 Asian School of the Environment, Nanyang Technological University, 50 Nanyang Avenue Singapore, Singapore 639798, Singapore; [email protected] 6 Division of Biological Sciences & Wildlife Biology Program, University of Montana, Missoula, MT 59812, USA; [email protected] 7 School of Biological and Environmental Sciences, Liverpool John Moores University, Exchange Station, Liverpool L2 2QP, UK; [email protected] * Correspondence: [email protected] Abstract: Logging and conversion of tropical forests in Southeast Asia have resulted in the expansion of landscapes containing a mosaic of habitats that may vary in their ability to sustain local biodiversity. Citation: Milne, S.; Martin, J.G.A.; However, the complexity of these landscapes makes it difficult to assess abundance and distribution Reynolds, G.; Vairappan, C.S.; Slade, of some species using ground-based surveys alone. -

An Assessment of Trade in Orang-Utans and Gibbons on Java and Bali,Indonesia
IN FULL SWING: AN ASSESSMENT OF TRADE IN ORANG-UTANS AND GIBBONS ON JAVA AND BALI,INDONESIA VINCENT NIJMAN A TRAFFIC SOUTHEAST ASIA REPORT TRAFFIC SOUTHEAST ASIA Published by TRAFFIC Southeast Asia, Petaling Jaya, Selangor, Malaysia © 2005 TRAFFIC Southeast Asia All rights reserved. All material appearing in this publication is copyrighted and may be produced with permission. Any reproduction in full or in part of this publication must credit TRAFFIC Southeast Asia as the copyright owner. The views of the authors expressed in this publication do not necessarily reflect those of the TRAFFIC Network, WWF or IUCN. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organizations concerning the legal status of any country, territory, or area, or its authorities, or concerning the delimitation of its frontiers or boundaries. The TRAFFIC symbol copyright and Registered Trademark ownership is held by WWF, TRAFFIC is a joint programme of WWF and IUCN. Layout by Noorainie Awang Anak, TRAFFIC Southeast Asia Suggested citation: Vincent Nijman (2005). In Full Swing: An Assessment of trade in Orang-utans and Gibbons on Java and Bali, Indonesia. TRAFFIC Southeast Asia ISBN 983-3393-00-4 Photograph credit: Orang-utan, Pongo pygmaeus, Sepilok Orang-utan Rehabilitation Centre, Sabah, Malaysia (WWF-Malaysia/Cede Prudente) IN FULL SWING: AN ASSESSMENT OF TRADE IN ORANG-UTANS AND GIBBONS ON JAVA AND BALI,INDONESIA -

Stolen Apes – the Illicit Trade in Chimpan- Zees, Gorillas, Bonobos and Orangutans
A RAPID RESPONSE ASSESSMENT STOLENTHE ILLICIT TRADE IN CHIMPANZEES, GORILLAS, BONOBOSAPES AND ORANGUTANS Stiles, D., Redmond, I., Cress, D., Nellemann, C., Formo, R.K. (eds). 2013. Stolen Apes – The Illicit Trade in Chimpan- zees, Gorillas, Bonobos and Orangutans. A Rapid Response Assessment. United Nations Environment Programme, GRID-Arendal. www.grida.no ISBN: 978-82-7701-111-0 Printed by Birkeland Trykkeri AS, Norway This publication was made possible through the financial UNEP and UNESCO support of the Government of Sweden. promote environmentally sound practices globally and in our own activi- Disclaimer ties. This publication is printed on fully recycled The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. The designations employed and the paper, FSC certified, post-consumer waste and presentations do not imply the expressions of any opinion whatsoever on chlorine-free. Inks are vegetable-based and coatings the part of UNEP or contributory organisations concerning the legal status of any country, territory, city, company or area or its authority, or concern- are water-based. Our distribution policy aims to ing the delimitation of its frontiers or boundaries. reduce our carbon footprint. STOLENTHE ILLICIT TRADE IN CHIMPANZEES, GORILLAS, BONOBOSAPES AND ORANGUTANS A RAPID RESPONSE ASSESSMENT Editorial Team Daniel Stiles Ian Redmond Doug Cress Christian Nellemann Rannveig Knutsdatter Formo Cartography Riccardo Pravettoni 4 PREFACE The trafficking of great apes adds additional and unwelcome pressures on charismatic fauna that provide an impetus for tourism and thus revenues to the economy. The illegal trade in wildlife makes up one part of the multi-billion dollar business that is environmental crime and is increasingly being perpetrated at the cost of the poor and vulnerable.