UPLAND DEPRESSION SWAMP FOREST Concept
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommended Trees for Winnetka
RECOMMENDED TREES FOR WINNETKA SHADE TREES Common_Name Scientific_Name Ohio Buckeye Acer galbra Miyabe Maple Acer miyabei Black Maple Acer nigrum Norway Maple Acer plantanoides v. ___ Sugar Maple (many cultivars) Acer saccharum Shangtung Maple Acer truncatum Autumn Blaze or Marmo Maple Acer x freemanii Red Horsechestnut Aesculus x carnea 'Briotii' Horsechestnut Aesulus hippocastanum Alder Alnus glutinosa Yellowwood Caldrastis lutea Upright European Hornbeam Carpinus betulus “Fastigata” American Hornbeam Carpinus carolinians Hickory Carya ovata Catalpa Catalpa speciosa Hackberry Celtis occidentalis Katsuratree Cercidiphyllum japonicum Turkish Filbert Corylus colurna American Beech Fagus grandifolia Green Beech Fagus sylvatica European Beech Fagus sylvatica Ginkgo Ginkgo biloba Thornless Honeylocust Gleditsia triacanthos inermis Kentucky Coffeetree Gymnocladus dioica Goldenraintree Koelreuteria paniculata Sweetgum Liquidambar styraciflua Tulip Tree Liriodendron tulipfera Black gum, Tupelo Liriodendron tulipfera Hophornbeam Ostrya virginiana Corktree Phellodendron amurense Exclamation Plantree Plantanus x aceerifolia Quaking Aspen Populus tremuloides Swamp White Oak Quercus bicolor Shingle Oak Quercus imbricaria Bur Oak Quercus macrocarpa Chinkapin Oak Quercus muehlenbergii English Oak Quercus robur Red Oak Quercus rubra Schumard Oak Quercus shumardii Black Oak Quercus velutina May 2015 SHADE TREES Common_Name Scientific_Name Sassafras Sassafras albidum American Linden Tilia Americana Littleleaf Linden (many cultivars) Tilia cordata Silver -

Quercus Phellos.Indd
Quercus phellos (Willow Oak) Beech Family (Fagaceae) Introduction: Willow oak is a member of the red oak group with willow-shaped leaves. The fi ne foliage of the willow oak is one of its best ornamental features. The willow oak has excellent texture, rounded form, attrac- tive bark, and beautiful winter features (fi ne-texture, persistent leaves and twiggy form). Culture: The willow oak is an excellent choice as a shade tree. It thrives in moist, well-drained, acidic soil and full sun. The willow oak will tolerate pollution and drought and is considered a trouble-free tree as long as soil pH is acidic. Willow oak has a fi brous root system and is therefore easy to transplant. It has no serious disease or Botanical Characteristics: insect problems. As little as 1 inch of fi ll soil can kill an oak. Native habitat: Southeastern USA in alluvial soils, swamps. Additional comments: Willow oak is an excellent large shade tree. Its Growth habit: Unique among the oaks, the wil- low oak has a rounded habit. fi ne texture contrasts with the coarseness of most other red oaks. It is one of the best oaks for avenue plantings Tree size: Growing fast for an oak, it can reach or large residences. Willow oak is a fast-growing oak 70’ in height and equal width. that transplants easily and is tolerant of a wide range of growing conditions. Flower and fruit: Female fl owers are incon- Willow oak is a member of the red oak group spicuous; however the pendulous male catkins without lobed leaves. -

Antimicrobial and Antioxidant Activity of the Leaves, Bark and Stems of Liquidambar Styraciflua L
Int.J.Curr.Microbiol.App.Sci (2016) 5(1): 306-317 ISSN: 2319-7706 Volume 5 Number 1(2016) pp. 306-317 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2016.501.029 Antimicrobial and Antioxidant Activity of the Leaves, Bark and Stems of Liquidambar styraciflua L. (Altingiaceae) Graziele Francine Franco Mancarz1*, Ana Carolina Pareja Lobo1, Mariah Brandalise Baril1, Francisco de Assis Franco2 and Tomoe Nakashima1 1Pharmaceutical ScienceDepartment, Universidade Federal do Paraná, Curitiba, PR, Brazil 2Coodetec Desenvolvimento, Produção e Comercialização Agrícola Ltda, Cascavel, PR, Brazil *Corresponding author A B S T R A C T K e y w o r d s The genus Liquidambar L. is the best-known genus of the Altingiaceae Horan family, and species of this genus have long been used for the Liquidambar treatment of various diseases. Liquidambar styraciflua L., which is styraciflua, popularly known as sweet gum or alligator tree, is an aromatic deciduous antioxidant tree with leaves with 5-7 acute lobes and branched stems. In the present activity, study, we investigated the antimicrobial and antioxidant activity of aerial antimicrobial parts of L.styraciflua. Antimicrobial activity was evaluated using the activity, microdilution methodology. The DPPH and phosphomolybdenum methods microdilution method, were used to assess the antioxidant capacity of the samples. The extracts DPPH assay showed moderate or weak antimicrobial activity. The essential oil had the lowest MIC values and exhibited bactericidal action against Escherichia Article Info coli, Enterobacter aerogenes and Staphylococcus aureus. The ethyl acetate fraction and the butanol fraction from the bark and stem showed the best Accepted: antioxidant activity. -

Native Trees of Georgia
1 NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION Eleventh Printing - 2001 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W. -

United States Department of Agriculture
Pest Management Science Pest Manag Sci 59:788–800 (online: 2003) DOI: 10.1002/ps.721 United States Department of Agriculture—Agriculture Research Service research on targeted management of the Formosan subterranean termite Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae)†‡ Alan R Lax∗ and Weste LA Osbrink USDA-ARS-Southern Regional Research Center, New Orleans, Louisiana, USA Abstract: The Formosan subterranean termite, Coptotermes formosanus Shiraki is currently one of the most destructive pests in the USA. It is estimated to cost consumers over US $1 billion annually for preventative and remedial treatment and to repair damage caused by this insect. The mission of the Formosan Subterranean Termite Research Unit of the Agricultural Research Service is to demonstrate the most effective existing termite management technologies, integrate them into effective management systems, and provide fundamental problem-solving research for long-term, safe, effective and environmentally friendly new technologies. This article describes the epidemiology of the pest and highlights the research accomplished by the Agricultural Research Service on area-wide management of the termite and fundamental research on its biology that might provide the basis for future management technologies. Fundamental areas that are receiving attention are termite detection, termite colony development, nutrition and foraging, and the search for biological control agents. Other fertile areas include understanding termite symbionts that may provide an additional target for control. Area-wide management of the termite by using population suppression rather than protection of individual structures has been successful; however, much remains to be done to provide long-term sustainable population control. An educational component of the program has provided reliable information to homeowners and pest-control operators that should help slow the spread of this organism and allow rapid intervention in those areas which it infests. -

Tree of the Year: Liquidambar Eric Hsu and Susyn Andrews
Tree of the Year: Liquidambar Eric Hsu and Susyn Andrews With contributions from Anne Boscawen (UK), John Bulmer (UK), Koen Camelbeke (Belgium), John Gammon (UK), Hugh Glen (South Africa), Philippe de Spoelberch (Belgium), Dick van Hoey Smith (The Netherlands), Robert Vernon (UK) and Ulrich Würth (Germany). Affinities, generic distribution and fossil record Liquidambar L. has close taxonomic affinities with Altingia Noronha since these two genera share gum ducts associated with vascular bundles, terminal buds enclosed within numerous bud scales, spirally arranged stipulate leaves, poly- porate (with several pore-like apertures) pollen grains, condensed bisexual inflorescences, perfect or imperfect flowers, and winged seeds. Not surpris- ingly, Liquidambar, Altingia and Semiliquidambar H.T. Chang have now been placed in the Altingiaceae, as originally treated (Blume 1828, Wilson 1905, Chang 1964, Melikan 1973, Li et al. 1988, Zhou & Jiang 1990, Wang 1992, Qui et al. 1998, APG 1998, Judd et al. 1999, Shi et al. 2001 and V. Savolainen pers. comm.). These three genera were placed in the subfamily Altingioideae in Hamamelidaceae (Reinsch 1890, Chang 1979, Cronquist 1981, Bogle 1986, Endress 1989) or the Liquidambaroideae (Harms 1930). Shi et al. (2001) noted that Altingia species are evergreen with entire, unlobed leaves; Liquidambar is deciduous with 3-5 or 7-lobed leaves; while Semiliquidambar is evergreen or deciduous, with trilobed, simple or one-lobed leaves. Cytological studies have indicated that the chromosome number of Liquidambar is 2n = 30, 32 (Anderson & Sax 1935, Pizzolongo 1958, Santamour 1972, Goldblatt & Endress 1977). Ferguson (1989) stated that this chromosome number distinguished Liquidambar from the rest of the Hamamelidaceae with their chromosome numbers of 2n = 16, 24, 36, 48, 64 and 72. -

Liquidambar Styraciflua L.) from Caroline County, Virginia
43 Banisteria, Number 9, 1997 © 1997 by the Virginia Natural History Society An Abnormal Variant of Sweetgum (Liquidambar styraciflua L.) from Caroline County, Virginia Bruce L. King Department of Biology Randolph Macon College Ashland, Virginia 23005 Leaves of individuals of Liquidambar styraciflua L. Similar measurements were made from surrounding (sweetgum) - are predominantly 5-lobed, occasionally 7- plants in three height classes:, early sapling, 61-134 cm; lobed or 3-lobed (Radford et al., 1968; Cocke, 1974; large seedlings, 10-23 cm; and small seedlings (mostly first Grimm, 1983; Duncan & Duncan, 1988). The tips of the year), 3-8.5 cm. All of the small seedlings were within 5 lobes are acute and leaf margins are serrate, rarely entire. meters of the atypical specimen and most of the large In 1991, I found a seedling (2-3 yr old) that I seedlings and saplings were within 10 meters. The greatest tentatively identified as a specimen of Liquidambar styrac- distance between any two plants was 70 meters. All of the iflua. The specimen occurs in a 20 acre section of plants measured were in dense to moderate shade. In the deciduous forest located between U.S. Route 1 and seedling classes, three leaves were measured from each of Waverly Drive, 3.2 km south of Ladysmith, Caroline ten plants (n = 30 leaves). In the sapling class, counts of County, Virginia. The seedling was found at the middle leaf lobes and observations of lobe tips and leaf margins of a 10% slope. Dominant trees on the upper slope were made from ten leaves from each of 20 plants (n include Quercus alba L., Q. -

Wood from Midwestern Trees Purdue EXTENSION
PURDUE EXTENSION FNR-270 Daniel L. Cassens Professor, Wood Products Eva Haviarova Assistant Professor, Wood Science Sally Weeks Dendrology Laboratory Manager Department of Forestry and Natural Resources Purdue University Indiana and the Midwestern land, but the remaining areas soon states are home to a diverse array reforested themselves with young of tree species. In total there are stands of trees, many of which have approximately 100 native tree been harvested and replaced by yet species and 150 shrub species. another generation of trees. This Indiana is a long state, and because continuous process testifies to the of that, species composition changes renewability of the wood resource significantly from north to south. and the ecosystem associated with it. A number of species such as bald Today, the wood manufacturing cypress (Taxodium distichum), cherry sector ranks first among all bark, and overcup oak (Quercus agricultural commodities in terms pagoda and Q. lyrata) respectively are of economic impact. Indiana forests native only to the Ohio Valley region provide jobs to nearly 50,000 and areas further south; whereas, individuals and add about $2.75 northern Indiana has several species billion dollars to the state’s economy. such as tamarack (Larix laricina), There are not as many lumber quaking aspen (Populus tremuloides), categories as there are species of and jack pine (Pinus banksiana) that trees. Once trees from the same are more commonly associated with genus, or taxon, such as ash, white the upper Great Lake states. oak, or red oak are processed into In urban environments, native lumber, there is no way to separate species provide shade and diversity the woods of individual species. -

Water's Park Tree Inventory and Assessment
Arborist’s Report Tree Inventory and Assessment 1 Waters Park Drive San Mateo, CA 94403 Prepared for: Strada Investment Group March 3, 2018 Prepared By: Richard Gessner ASCA - Registered Consulting Arborist ® #496 ISA - Board Certified Master Arborist® WE-4341B ISA - Tree Risk Assessor Qualified CA Qualified Applicators License QL 104230 © Copyright Monarch Consulting Arborists LLC, 2018 1 Waters Park Drive, San Mateo Tree Inventory and Assessment March 3, 2018 Table of Contents Summary............................................................................................................... 1 Introduction........................................................................................................... 2 Background ............................................................................................................2 Assignment .............................................................................................................2 Limits of the assignment ........................................................................................2 Purpose and use of the report ................................................................................3 Observations......................................................................................................... 3 Tree Inventory .........................................................................................................3 Analysis................................................................................................................ -

Phylogeographical Structure of Liquidambar Formosana Hance Revealed by Chloroplast Phylogeography and Species Distribution Models
Article Phylogeographical Structure of Liquidambar formosana Hance Revealed by Chloroplast Phylogeography and Species Distribution Models 1,2, 1, 1 2 1 1, Rongxi Sun y , Furong Lin y, Ping Huang , Xuemin Ye , Jiuxin Lai and Yongqi Zheng * 1 State Key Laboratory of Tree Genetics and Breeding, Key Laboratory of Silviculture of the State Forestry Administration, Research Institute of Forestry, Chinese Academy of Forestry, Beijing 100091, China; [email protected] (R.S.); [email protected] (F.L.); [email protected] (P.H.); [email protected] (J.L.) 2 Jiangxi Provincial Key Laboratory of Silviculture, College of Forestry, Jiangxi Agricultural University, Nanchang 330045, China; [email protected] * Correspondence: [email protected]; Tel.: +86-10-6288-8565 These authors contributed equally to this work. y Received: 2 September 2019; Accepted: 29 September 2019; Published: 1 October 2019 Abstract: To understand the origin and evolutionary history, and the geographical and historical causes for the formation of the current distribution pattern of Lquidambar formosana Hance, we investigated the phylogeography by using chloroplasts DNA (cpDNA) non-coding sequences and species distribution models (SDM). Four cpDNA intergenic spacer regions were amplified and sequenced for 251 individuals from 25 populations covering most of its geographical range in China. A total of 20 haplotypes were recovered. The species had a high level of chloroplast genetic variation (Ht = 0.909 0.0192) and a significant phylogeographical structure (genetic differentiation takes into ± account distances among haplotypes (Nst) = 0.730 > population differentiation that does not consider distances among haplotypes (Gst) = 0.645; p < 0.05), whereas the genetic variation within populations (Hs = 0.323 0.0553) was low. -
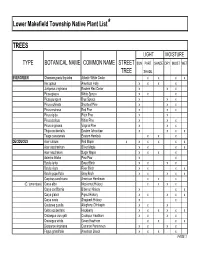
Native Plant List Trees.XLS
Lower Makefield Township Native Plant List* TREES LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE EVERGREEN Chamaecyparis thyoides Atlantic White Cedar x x x x IIex opaca American Holly x x x x Juniperus virginiana Eastern Red Cedar x x x Picea glauca White Spruce x x x Picea pungens Blue Spruce x x x Pinus echinata Shortleaf Pine x x x Pinus resinosa Red Pine x x x Pinus rigida Pitch Pine x x Pinus strobus White Pine x x x Pinus virginiana Virginia Pine x x x Thuja occidentalis Eastern Arborvitae x x x x Tsuga canadensis Eastern Hemlock xx x DECIDUOUS Acer rubrum Red Maple x x x x x x Acer saccharinum Silver Maple x x x x Acer saccharum Sugar Maple x x x x Asimina triloba Paw-Paw x x Betula lenta Sweet Birch x x x x Betula nigra River Birch x x x x Betula populifolia Gray Birch x x x x x Carpinus caroliniana American Hornbeam x x x (C. tomentosa) Carya alba Mockernut Hickory x x x x Carya cordiformis Bitternut Hickory x x x Carya glabra Pignut Hickory x x x x x Carya ovata Shagbark Hickory x x Castanea pumila Allegheny Chinkapin xx x Celtis occidentalis Hackberry x x x x x x Crataegus crus-galli Cockspur Hawthorn x x x x Crataegus viridis Green Hawthorn x x x x Diospyros virginiana Common Persimmon x x x x Fagus grandifolia American Beech x x x x PAGE 1 Exhibit 1 TREES (cont'd) LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE DECIDUOUS (cont'd) Fraxinus americana White Ash x x x x Fraxinus pennsylvanica Green Ash x x x x x Gleditsia triacanthos v. -

Quercus Lyrata Walt. Overcup Oak Fagaceae Beech Family J
Quercus lyrata Walt. Overcup Oak Fagaceae Beech family J. D. Solomon Overcup oak (Quercus Zyratu), also called swamp Habitat post oak, swamp white oak, and water white oak, is quite tolerant of flooding and grows slowly on poorly Native Range drained flood plains and swamp lands of the Southeastern United States. It may take 30 years Overcup oak (fig. 1) inhabits the wetter sites in before overcup oak produces acorns. Wildlife use bottom lands of the Coastal Plain from Delaware and them as food. The quality of the lumber varies great- Maryland south to Georgia and northwestern ly and the wood may check and warp during season- Florida; west to eastern Texas. It grows northward ing. It is cut and sold as white oak. in the Mississippi Valley to southeastern Oklahoma, Figure l-The native range of overcup oak. The author is Principal Research Entomologist, Southern Forest Experiment Station, New Orleans, LA. 681 Quercus lyrata southeastern Missouri, southern Illinois, south- endure submergence from late spring floods. In tests, western Indiana, and western Kentucky (8). overcup oak survived continuous flooding for at least two growing seasons. In spite of its natural occur- Climate rence on wet clay sites, overcup oak grows best on sites with better drainage and soil texture (10). The climate is warm and humid throughout the range of overcup oak (10). In the region where the Associated Forest Cover species grows best, total precipitation averages 1140 to 1520 mm (45 to 60 in) per year of which 510 to Overcup oak is usually a dominant species only in 760 mm (20 to 30 in) is received during the April-to- the forest cover type Overcup Oak-Water Hickory September growing season.