Effects of Crude Oil on Survival and Development in Embryonated Eggs in Callinectes Sapidus Rathbun, 1896 (Decapoda, Portunidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Duck Island Yacht Club 2017 N 41° 16.6’ W 72° 28.0’
DUCK ISLAND YACHT CLUB 2017 N 41° 16.6’ W 72° 28.0’ Founded 1932 Check out Cindy’s New Location 688 Boston Post Road in Westbrook, CT 860-399-0007 Mon-Thurs 9am-8pm Fri & Sat 9 am-9pm Sun 10am-6pm [email protected] We deliver locally! We offer a wide variety of wines from around the world. Our coolers host a range of beer, ale, lager, IPA, ciders, including brands brewed here in CT! If we don’t have what you want- we can order it and have it to you in two days! We also make gift baskets to order. Join us Friday evenings from 6-8 pm for wine and beer tastings! Bob Connell – Manager – North Yard Tel: 860 399-5128 Fax: 860 399-8720 Email: [email protected] 2. Connecticut Yacht Rigging Connecticut Yacht Rigging is dedicated to bringing you the highest quality rigging work with unsurpassed customer support. We have over 30 years of experience in marine, architectural and custom rig- ging. Additionally, we are Navtec factory trained in hydraulic installation and repair. Our exceptional attention to detail guar- antees your project is fulfilled accurately and on schedule. Connecticut Yacht Rigging provides a wide Contact Us Today: range of yacht rigging services including: Dan Coan, owner 860-575-6985 • Inspections & Insurance Claims [email protected] • Offshore Preparation • Mainsail Handling & Furling Systems • Jib Furling Systems • Downwind Gear • Standing & Running Rigging • Anchoring Systems • Hydraulic Systems • Bowsprits • Mast Refurbishing • Deck Hardware • Custom Hardware, Mast Collars & Radar Brackets • Radar Mast and Arch Installation • Hydraulic installation and repair 3. -
Ollys Comet Toocooltoofool Missile
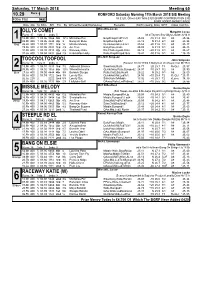
Saturday, 17 March 2018 Meeting 65 10.29 Race 1 ROMFORD Saturday Morning 17th March 2018 SIS Meeting 400m Flat (A8) 1st £120, Others £40 Total £320 (BGRF CONTRIBUTION £30) BGRF ADDED MONEY RACE Date Dis Tp STm SPl Fin By Winner/Second(Hcp/Venue) Remarks WinTm Going Kilos SP/T Class CalcTm Miss.M.E.Lucas OLLYS COMET Margaret Lucas Runs: 37 1sts: 3 2nds: 5 bk d Taylors Sky-Ollys Louise Jy'14 Ir 1 12.Mr 400 1 04.06 3366 5th 12¼ Michelles Pet BmpRnUp,FcdTCk¼ 25.08 -10 31.4 9/2 A8 25. 96 03.Mr 400 1 03.95 4544 4th 3 Bang On Elsie BmpRunUp,Blk1 25.13 N 31.4 6/1 A8 25. 38 Red 26.Fb 400 1 03.97 1111 3rd ¾ Missile Melody QuickAw,Rails,LdTo4 25.62 -20 31.6 7/2 A8 25. 47 19.Fb 400 2 04.05 3334 3rd 4¼ Act Tres EarlyPace,Rails 25.09 N 31.9 3/1 A8 25. 43 10.Fb 400 1 03.98 3313 4th 4¼ Raceway Katie Rls,Challenged3-RnIn 25.12 +20 31.5 3/1 A8 25. 67 31.Ja 400 1 04.04 5432 2nd 2¼ Knockalton Holly Rails,BmpRnUp&1&¾ 25.41 -30 31.6 5/1 A8 *25. 29 Mrs.M.E.Simpson TOOCOOLTOOFOOL John Simpson Runs: 13 1sts: 0 2nds: 3 (Season 10.Oc'17)bk b Ballymac Vic-Royal Visit Mr'15 Ir 2 13.Mr 400 1 04.01 3333 3rd 7½ Adamant Brienne SlowAway,Rails 24.77 +20 25.7 T3 ReQul 25. -

Designing a Smartphone Application for Supporting the COMET-Program in Mental Health Care
1 Faculty of Electrical Engineering, Mathematics & Computer Science Designing a smartphone application for supporting the COMET-program in mental health care Bob Loos Master Thesis Interaction Technology July 2020 Supervisors: prof.dr. D.K.J. Heylen (HMI) dr. R. Klaassen (HMI) dr. K.P. Truong (HMI) drs. Y.P.M.J. Derks (PHT) Human Media Interaction Group University of Twente Disclaimer This research is based on the COMET-methodology as designed by Korrelboom (2000). Apart from COMET being used, this research is not connected to any other research by Korrelboom. The emphasis of this research is laid on the design of a con- cept of a mobile application. This does not include the effectiveness of the latter on the patient's progress. On 17-02-2020, Korrelboom has given explicit permission for using the COMET-methodology in the current publication as well as for making the used prototypes available to the public. In this publication and in the prototypes, two screenshots from videos from VGCt and Gedachten Uitpluizen are used. On 23-04-2020 explicit permission for using these materials is granted. Furthermore, freely available material is used from svgrepo.com, the Spotify Branding Guideline and Pexels.com. Where possible, attribution is provided. The prototype does not contain any content that is copyrighted (unless permission is granted by the relevant party). Patients from mental healthcare organisation GGNet Doetinchem are asked to par- ticipate. During this part of the research, the researcher was following an internship at GGNet Doetinchem. Nor does this report or the prototypes contain patient information. For this research, permission of the Ethical Committee EWI of the University of Twente has been requested and granted on 07-08-2019. -

October 2015 Enewsletter
From the President’s Desk This has been another active season for the Comet Class. Since the last E-New there have been four successful regattas: Corsica River Annual Regatta, the Junior Championship, the Internationals, and the Pigskin Regatta. Information regarding them is to follow. A new Comet fleet is in the making down in Hampton, Virginia and they are looking to grow their fleet. We welcome the new owners Scott Wolff and Kathy Barber. Many thanks are extended to Andy Wood for his diligent commitment to running both the North Americans and the International Championships. The time and effort of sending many emails, making many phone calls, revision of both the NORs and the SIs is greatly appreciated. For his E-NEWS dedication, he was again awarded the Presidents Cup. This year the Citizens Cup was awarded to Lee Ingram for all her contributions to the Class. She has been willing and able to give guidance October and assistance to myself, Brad, and many others regardless of the task 2015 at hand. Whitecap Composites has now completed the new hull mold and are in the www.cometclass.com process of making their first Comet. Lee and I plan to test sail her before going down to the Pumpkin Bowl November 7. Come down to check out the new Comet. News about our sailors Thanks again to the many donors that made the new hull mold possible. Thanks to Brad Meade for his expertise in spear heading the technical Chris Price and Amanda Reynold aspects with the builders. Thanks to Dick Harmon and Kevin Buruchian were married on May 29th for always being available to assist on this project. -

2019 One Design Classes and Sailor Survey
2019 One Design Classes and Sailor Survey [email protected] One Design Classes and Sailor Survey One Design sailing is a critical and fundamental part of our sport. In late October 2019, US Sailing put together a survey for One Design class associations and sailors to see how we can better serve this important constituency. The survey was sent via email, as a link placed on our website and through other USSA Social media channels. The survey was sent to our US Sailing members, class associations and organizations, and made available to any constituent that noted One-Design sailing in their profile. Some interesting observations: • Answers are based on respondents’ perception of or actual experience with US Sailing. • 623 unique comments were received from survey respondents and grouped into “Response Types” for sorting purposes • When reviewing data, please note that “OTHER” Comments are as equally important as those called out in a specific area, like Insurance, Administration, etc. • The majority of respondents are currently or have been members of US Sailing for more than 5 years, and many sail in multiple One-Design classes • About 1/5 of the OD respondents serve(d) as an officer of their primary OD class; 80% were owner/drivers of their primary OD class; and more than 60% were members of their primary OD class association. • Respondents to the survey were most highly concentrated on the East and West coasts, followed by the Mid- West and Texas – though we did have representation from 42 states, plus Puerto Rico and Canada. • Most respondents were male. -

Classic Lightning from the Desk of the Class Historian Corky Gray
Classic Lightning From the Desk of the Class Historian Corky Gray The NOMINATION of JOHN S. BARNES for the NATIONAL SAILING HALL of FAME The Executive Board of the International Lightning Class Association has approved a nomination to the National Sailing Hall of Fame for our founding father, John S. Barnes. Cited as an early promoter of one-design sailboat racing and founder of the Lightning Class, he is responsible for the establishment of the first high- volume production manufacturing company of one-design racing sailboats. Barnes is also recognized for the development and patenting of a vacuum bag molding process for sailboat production. Barnes has joined the queue, along with Tom Allen, Ed Adams, Bob Bavier, Jim Carson, Dave Dellenbaugh, Skip Etchells, Greg Fisher, Marty O’Meara, and Brad and Ken Reed, to join the fifteen Lightning Class members already inducted into the National Sailing Hall of Fame. Barnes was born in 1905 to A. E. “Skipper” and Eva The idea of racing small sailboats of a single design Snaith Barnes of Syracuse, New York. His father evolved in the early twentieth century. It was common earned a degree in engineering from Cornell and for individual sailing clubs to have small keelboats prospered in the business world. His success enabled as a one-design fleet for local competition. The idea of him to buy a summer retreat in Henderson Harbor on a single design to be raced regionally or nationwide Lake Ontario, where he based his forty-foot yawl ‘The- was new. The Star Class in 1911 was the first class to become a class raced in many different parts of the mis.’ Henderson Harbor was a summer home to many country. -

ENSIGN FLEET Back in Drought Conditions in Central Texas, and the Lake Is Going Down Pretty Rapidly
AUSTIN YACHT CLUB TELLTALE August 2020 Sailing during Covid-19 Stay safe, stay healthy! Message from the Commodore It is August, it is HOT. We are FEATURED: ENSIGN FLEET back in drought conditions in Central Texas, and the lake is going down pretty rapidly. Russ and the Dock Crew will start moving docks to their first low water positions very soon. Fortunately, there is still plenty of water for sailing and racing. It is great to see the fleets out on Wednesday evenings and on the weekends in their informal racing. The wind has been great this summer! The Board and the Covid Advisory Committee have been working hard to develop a roadmap to reopening the Club. That roadmap will have been sent out to every member via email and will have been posted on the website. It is also included in this Telltale. The roadmap lists which activities and areas are available with what conditions at each Covid stage. The stage we are in is determined by Austin and Travis County. Please be aware that this is a living document. It is constantly under review and may change as conditions and information change. We are hopeful that the downward trend in Covid cases continues and we look forward to opening up more of the Club as it does. Again, a big thank you to the Board and the Covid Advisory Committee for all their hard work. Annie and Charlie Lancaster on Eagle, 2019 Ensign Regionals Photo by Bill Records New member Jeffrey Lane and son Colt sailing their just launched Pearson 26OD Photo by Bill Records IN THIS ISSUE Heave-to/Boat Docking by Harry Polly -

Seafood Tower Foie Gras 5 | Bleu Cheese 3 | Truffled 3 the Collection of Seafood Below Is Either Chilled Or Baked
SOCIAL hour 5pm - 7pm daily RAW/CHILLED EAST & WEST COAST OYSTERS 3.50 ea PRIME CUTS Changes daily, please inquire PETITE FILET MIGNON 8oz (21 Day) 42 FILET MIGNON 12oz (21 Day) 55 GIANT SHRIMP COCKTAIL 9 ea Bloody Mary Cocktail Sauce BONE-IN RIBEYE 22oz (35 Day) 59 NEW YORK STRIP 14oz (35 Day) 49 ALASKAN KING CRAB AQ DELMONICO 18oz (35 Day Dry-Aged) 69 COLOSSAL BLUE CRAB 21 PORTERHOUSE 36oz (35 Day - For Two) AQ TUNA TARTARE 18 VEAL CHOP 16oz 58 Golden Beets, Avocado & Thai Chiles CEVICHE 14 Steak Sauces (Complimentary) Local Mango, Avocado, Jicama & Aji Limo CUT 432 Steak Sauce, Bearnaise, Creamy Horseradish Toasted Peppercorn & Chimichurri SAUCES Cocktail Sauce | Hot Sauce | Apple-Cucumber Mignonette | Drawn Butter Accompaniments Hudson Valley Foie Gras 16 | King Crab Oscar 18 Butters Seafood Tower Foie Gras 5 | Bleu Cheese 3 | Truffled 3 The collection of seafood below is either chilled or baked BAKED AQ Jumbo Lump Crabcakes, Oysters Rockefeller, Shrimp Scampi & South African Lobster Tail CHILLED ENTRÉES East & West Coast Oysters, Giant Shrimp, Alaskan King Crab & Ceviche CHEESESBURGER 18 Jackman Ranch Wagyu, Aged Vermont Cheddar & Hand-Cut Fries S TA R T E R S ROASTED HALF CHICKEN 29 Prosciutto Wrapped Asparagus, Fingerling Potatoes & Pan Jus OYSTERS ROCKEFELLER 17 Blue Point Oysters, Spinach Mornay & Crispy Pancetta BERKSHIRE PORK CHOP 38 STEAK TARTARE 21 Vermont Maple Spiked Sweet Potatoes, Medjool Date Verjus Cornichon, Shallot, Capers, Preserved Lemon & Quail Egg Persimmons & Baby Kale BRAISED SHORT RIB MEATBALLS 17 San Marzano -

2018 Charity Boat Auction Inventory Thank You for Your Generous
Thank you for your generous support of the Chesapeake Bay Maritime Museum ! 2018 Charity Boat Auction Inventory INV # DESCRIPTION TYPE MACGREGOR 26. 1987. Iconic trailerable weekender w/ 9.9 hp Honda 4 stroke o/b motor and good tandem axle 5009 untitled storage trailer. Fantastic bay and inland cruiser for most anywhere you can haul and launch her. Sea Sail of Cortez anyone ? Untitled storage trailer included. MD 6620 CH. CURRENT DESIGNS FITNESS KAYAK. Freedom model. 18 ft. long and 21 3/4 beam. Only 33 lbs. ! Kevlar 5019 construction with rudder and adjustable seat. As new condition . No paddle. Untitled, unregistered smallcraft not Paddle intended for motorization. AMF SUNFISH. 1969 Original owner boat used exclusively on fresh water lake in PA. Green stripe and splash 5031 guard. Well cared for and complete boat ready for more fun. Everyone loves a Sunfish. Why not treat yourself or Sail your kids to one. Untitled, unregistered smallcraft not intended for motorization. MISTRAL WINDSURFER. Really nice condition! Mast, boom, two sails (one brand new!), and sailbag included. 5038 Sail Untitled, unregistered smallcraft. BOMBARDIER SEADOO CHALLENGER 1800. 1997 twin Rotax water jet sport boat with bimini top. Motors need 5045 attention / replacement, jet pumps appear sound. Good project / parts boat, or buy it for the very nice galvanized, Power titled Sea Doo trailer. MD 3421 BJ. CAPE COD SENIOR KNOCKABOUT. Beautiful 23 ft. Spaulding Dunbar design built by Cape Cod Shipbuilding 1940's. Graceful c/b sloop with large cockpit and simple rig. Quite similiar to a Sakonnet 23 with a counter stern, W Class 22, Hodgon 21, etc.. -

Rock Hall Yacht Club 2018 One-Design Regatta, Saturday and Sunday, June 16-17, 2018 Windmill District Championship Wayfarer North Americans Comet North Americans
Rock Hall Yacht Club 2018 One-Design Regatta, Saturday and Sunday, June 16-17, 2018 Windmill District Championship Wayfarer North Americans Comet North Americans Course A (Entries=36) Comets - Wayfarer – Windmill Principal Race Officer: Bill Colgan Division: Comets (13 boats) Total Pos Sail Skipper Boat Club 1 2 3 4 Pos Points 1 4104 DAN CURRAN Laney Errickson Riverton y.c. 7 2 3 1 13.00 1 2 4151 Talbott Ingram Mel Keen Shrewsbury Sailing & Yacht Club 4 6 1 4 15.00 2 3 4087 Andrew Wood Abby Silva Corsica River Yacht Club 2 4 5 7 18.00 3 4 4037 Elliott Oldak Barbara Best Severn Sailing Association 1 1 14/OCS 3 19.00 4 5 4137 Joe Lauver Ian Lauver Susquehanna Yacht Club 3 3 14/OCS 2 22.00 5 6 3957 Chris Price Sr Potapskut Sailing Assoc 8 7 6 5 26.00 6 7 3881 John Ebken Laura Verdi SDYC 9 10 2 8 29.00 7 8 3952 Mark Buruchian Shannon Buruchian Highland Lakes Comet Fleet #130 10 5 4 11 30.00 8 9 4069 Art Silcox Karen Glass Corsica River YC 6 9 9 6 30.00 9 Total Pos Sail Skipper Boat Club 1 2 3 4 Pos Points 10 4090 Samuel Turvey John Wallace Shrewsbury Sailing and Yacht Center 5 11 7 10 33.00 10 11 4152 Tom Layton Jack Bruning Niantic Bay Yacht Club 11 8 8 9 36.00 11 12 4096 Mike Hollis Kelly Girandola Corsica River 13 13 10 14/DNF 50.00 12 13 4089 Scott Wolff Broad Bay Sailing Association 12 12 14/DNF 14/DNF 52.00 13 Division: Wayfarer (10 boats) Total Pos Sail Skipper Boat Club 1 2 3 4 Pos Points 1 11221 Marc Bennett Julie Saraphinoff LSC 1 3 1 1 6.00 1 2 10962 Mike Duncan Margaret Duncan Mississauaga Sailing Club 2 1 2 5 10.00 2 3 3854 AL -

(2014) Assessment of Blue Swimmer Crab Recruitment
Fisheries Research Report No. 258, 2014 Assessment of blue swimmer crab recruitment and breeding stock levels in the Peel-Harvey Estuary and status of the Mandurah to Bunbury Developing Crab Fishery D. Johnston, A. Chandrapavan, B. Wise, N. Caputi Fisheries Research Division Western Australian Fisheries and Marine Research Laboratories PO Box 20 NORTH BEACH, Western Australia 6920 1390/14 Correct citation: Johnston, D., Chandrapavan, A., Wise, B. and Caputi, N. 2014. Assessment of blue swimmer crab recruitment and breeding stock levels in the Peel-Harvey Estuary and status of the Mandurah to Bunbury Developing Crab Fishery. Fisheries Research Report No. 258. Department of Fisheries, Western Australia. 148pp. Enquiries: WA Fisheries and Marine Research Laboratories, PO Box 20, North Beach, WA 6920 Tel: +61 8 9203 0111 Email: [email protected] Website: www.fish.wa.gov.au ABN: 55 689 794 771 A complete list of Fisheries Research Reports is available online at www.fish.wa.gov.au © Department of Fisheries, Western Australia. October 2014. ISSN: 1035 - 4549 ISBN: 978-1-921845-80-2 ii Fisheries Research Report [Western Australia] No. 258, 2014 Contents Executive Summary ............................................................................................................. 1 1.0 General Introduction .................................................................................................. 5 1.1 Physical description of Peel-Harvey Estuary ......................................................... 5 1.2 Peel-Harvey Estuary commercial -

75Th CRYC Annual Regatta One-Design Notice of Race CORSICA RIVER YACHT CLUB
75th CRYC Annual Regatta One-Design Notice Of Race CORSICA RIVER YACHT CLUB Saturday 23 July 2016 Sunday 24 July 2016 Entry Due: Saturday 23 July 2016 (from 0900-1100). Entry Fee: CBYRA Members: $25 On-site entry available starting 0830 @ CRYC Ship Point Others: $35 On-site: (no discounts) $40 Online Entry: Check the CRYC web site at www.cryc.org for online entry. Mail To: N/A Online or on-site registration only. Contact: John Foster: 443-496-0291, email: [email protected] Eligible: Laser, Laser Radial, 420, Comet, Windmill, Hampton, Penguin, Catboats, Beach Cats, Optimist (all fleets). Other classes may be invited (see www.cryc.org for details). Rendezvous: Onshore at Corsica River Sailing Center Skippers’ Meeting. Start: Between orange flag on Race Committee boat and mark representing the start pin. See Event Sailing Instructions for more detail at www.cryc.org. Sequence will be set on each day of racing. Signals: RRS Rule 26 (Starting Races) applies. Saturday 0930 Skippers Meeting 1030 Harbor Gun 1200 1st Warning; Sunday 930 Skippers Meeting, 1000 Harbor Gun, 1100 1st warning. Lunches available for purchase on race days. Course: Drop mark type courses or as designated by letters displayed on Race Committee boat. Two or three racing areas may be used each using different style marks. Marks will be consistent within the racing areas. Finish: Between orange flag on Race Committee boat and mark representing the finish pin. See Event Sailing Instructions for more detail at www.cryc.org. Rules: This regatta will be governed by the ‘rules’ as defined in The Racing Rules of Sailing 2013-2016 (RRS).