Que Tempolate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -

Multicatalytic, Asymmetric Michael/Stetter Reaction Of
Multicatalytic, asymmetric Michael/Stetter reaction SPECIAL FEATURE of salicylaldehydes and activated alkynes Claire M. Filloux, Stephen P. Lathrop, and Tomislav Rovis1 Department of Chemistry, Colorado State University, Fort Collins, CO 80523 Edited by David W. C. MacMillan, Princeton University, Princeton, NJ, and accepted by the Editorial Board May 30, 2010 (received for review March 22, 2010) We report the development of a multicatalytic, one-pot, asymmetric Ar Ar Michael/Stetter reaction between salicylaldehydes and electron- N H deficient alkynes. The cascade proceeds via amine-mediated OO 3 OTMS (20 mol %) Me HO Me Michael addition followed by an N-heterocyclic carbene-promoted Me Me 1 Ar = 3,5-(CF3)2C6H3 O intramolecular Stetter reaction. A variety of salicylaldehydes, dou- + O O BF4 N bly activated alkynes, and terminal, electrophilic allenes participate 2 Me 5 Me in a one-step or two-step protocol to give a variety of benzofura- NNC F 4a 6 5 One-Pot Two - Po t none products in moderate to good yields and good to excellent (10 mol %) 93% yield 46% yield NaOAc (10 mol %) 85:15:<1:<1 dr 5:1 dr enantioselectivities. The origin of enantioselectivity in the reaction 86% ee 58% ee CHCl3, 23 C is also explored; E∕Z geometry of the reaction intermediate as well as the presence of catalytic amounts of catechol additive are O found to influence reaction enantioselectivity. O Me * O Me ∣ ∣ catalysis organic synthesis tandem catalysis Me 6 ascade catalysis has garnered significant recent attention Scheme 1. Multicatalytic Michael/Benzoin cascade. Cfrom the synthetic community as a means to swiftly assemble CHEMISTRY complex molecules from simple starting materials with minimal benzofuranones. -

Article 22 Regulation for Restriction of Synthetic Drugs
ARTICLE 22 REGULATION FOR RESTRICTION OF SYNTHETIC DRUGS SECTION 22.1 AUTHORITY This regulation is promulgated under the authority granted to the Needham Board of Health under Massachusetts General Laws Chapter 111, Section 31 which states that “boards of health may make reasonable health regulations”. SECTION 22.2 PURPOSE The Needham Board of Health has found that synthetic marijuana, consisting of plant or other material treated with various chemicals or other synthetic substances not approved for human consumption, may be marketed and sold as herbal incense in the greater Boston area, although they are being used in the same manner and for the same purposes as scheduled drugs. In addition, the use of these products has become particularly popular among teens and young adults. Based on information and reports from hospitals, emergency room doctors, and police agencies, individuals who use these products experience dangerous side effects including convulsions, hallucinations, and dangerously elevated heart rates. This is evidence that synthetic marijuana products are harmful if inhaled or consumed, and present a significant public health danger. These synthetic compounds and others have a high potential for abuse and lack of any accepted medical use, these dangerous products, while not approved for human consumption, are marketed and sold in a form that allows for such consumption, putting at risk the individuals who come into contact with them. Therefore, the Needham Board of Health adopts this regulation for the purpose and with the intent to protect the public health and safety of the Town of Needham and its residents from the threat posed by the availability and use of synthetic marijuana, synthetic stimulants, synthetic hallucinogens, and other dangerous products by prohibiting persons from trafficking in, possessing, and using them within the town. -

2 Spice English Presentation
Spice Spice contains no compensatory substances Специи не содержит компенсационные вещества Spice is a mix of herbs (shredded plant material) and manmade chemicals with mind-altering effects. It is often called “synthetic marijuana” because some of the chemicals in it are similar to ones in marijuana; but its effects are sometimes very different from marijuana, and frequently much stronger. It is most often labeled “Not for Human Consumption” and disguised as incense. Eliminationprocess • The synthetic agonists such as THC is fat soluble. • Probably, they are stored as THC in cell membranes. • Some of the chemicals in Spice, however, attach to those receptors more strongly than THC, which could lead to a much stronger and more unpredictable effect. • Additionally, there are many chemicals that remain unidentified in products sold as Spice and it is therefore not clear how they may affect the user. • Moreover, these chemicals are often being changed as the makers of Spice alter them to avoid the products being illegal. • To dissolve the Spice crystals Acetone is used endocannabinoids synhtetic THC cannabinoids CB1 and CB2 agonister Binds to cannabinoidreceptor CB1 CB2 - In the brain -in the immune system Decreased avtivity in the cell ____________________ Maria Ellgren Since some of the compounds have a longer toxic effects compared to naturally THC, as reported: • negative effects that often occur the day after consumption, as a general hangover , but without nausea, mentally slow, confused, distracted, impairment of long and short term memory • Other reports mention the qualitative impairment of cognitive processes and emotional functioning, like all the oxygen leaves the brain. -

Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected]
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 4-25-2018 Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances Joshua Zolton Seither [email protected] DOI: 10.25148/etd.FIDC006565 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Chemistry Commons Recommended Citation Seither, Joshua Zolton, "Application of High Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances" (2018). FIU Electronic Theses and Dissertations. 3823. https://digitalcommons.fiu.edu/etd/3823 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida APPLICATION OF HIGH RESOLUTION MASS SPECTROMETRY FOR THE SCREENING AND CONFIRMATION OF NOVEL PSYCHOACTIVE SUBSTANCES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Joshua Zolton Seither 2018 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Joshua Zolton Seither, and entitled Application of High- Resolution Mass Spectrometry for the Screening and Confirmation of Novel Psychoactive Substances, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Piero Gardinali _______________________________________ Bruce McCord _______________________________________ DeEtta Mills _______________________________________ Stanislaw Wnuk _______________________________________ Anthony DeCaprio, Major Professor Date of Defense: April 25, 2018 The dissertation of Joshua Zolton Seither is approved. -

Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)
Model Scheduling New/Novel Psychoactive Substances Act (Third Edition) July 1, 2019. This project was supported by Grant No. G1799ONDCP03A, awarded by the Office of National Drug Control Policy. Points of view or opinions in this document are those of the author and do not necessarily represent the official position or policies of the Office of National Drug Control Policy or the United States Government. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws. Please contact NAMSDL at [email protected] or (703) 229-4954 with any questions about the Model Language. This document is intended for educational purposes only and does not constitute legal advice or opinion. Headquarters Office: NATIONAL ALLIANCE FOR MODEL STATE DRUG 1 LAWS, 1335 North Front Street, First Floor, Harrisburg, PA, 17102-2629. Model Scheduling New/Novel Psychoactive Substances Act (Third Edition)1 Table of Contents 3 Policy Statement and Background 5 Highlights 6 Section I – Short Title 6 Section II – Purpose 6 Section III – Synthetic Cannabinoids 13 Section IV – Substituted Cathinones 19 Section V – Substituted Phenethylamines 23 Section VI – N-benzyl Phenethylamine Compounds 25 Section VII – Substituted Tryptamines 28 Section VIII – Substituted Phenylcyclohexylamines 30 Section IX – Fentanyl Derivatives 39 Section X – Unclassified NPS 43 Appendix 1 Second edition published in September 2018; first edition published in 2014. Content in red bold first added in third edition. © 2019 NATIONAL ALLIANCE FOR MODEL STATE DRUG LAWS. This document may be reproduced for non-commercial purposes with full attribution to the National Alliance for Model State Drug Laws. -

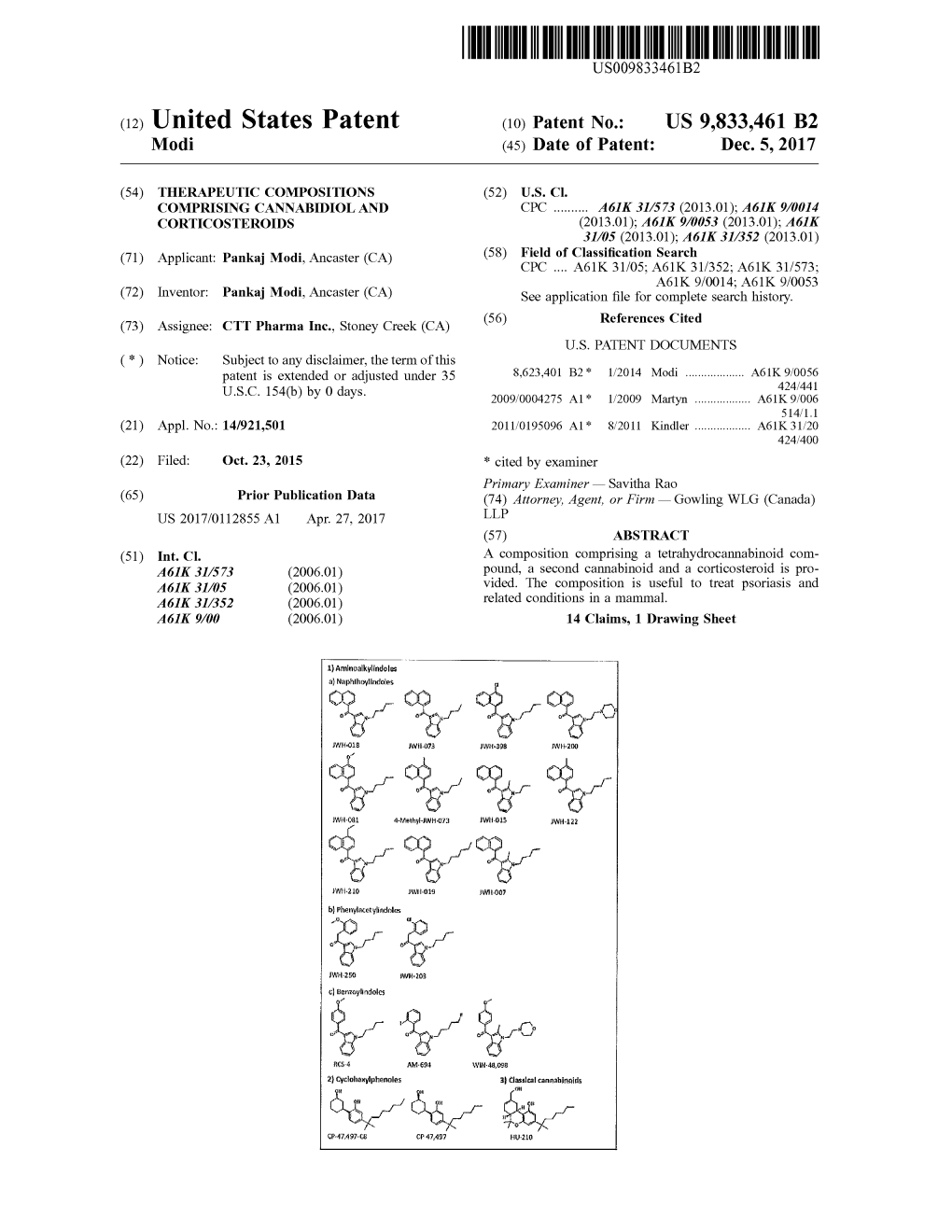
(12) Patent Application Publication (10) Pub. No.: US 2012/0321727 A1 Windschauer Et Al
US 20120321727A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0321727 A1 Windschauer et al. (43) Pub. Date: Dec. 20, 2012 (54) TASTE MASKING COMPOSITIONS AND A636/54 (2006.01) EDIBLE FORMS THEREOF A6II 3/40 (2006.01) A6II 3L/225 (2006.01) (75) Inventors: Robert J. Windschauer, Largo, FL A6IP 5/00 (2006.01) (US); Teresa T. Virgallito, A2.3L. I./22 (2006.01) Beavercreek, OH (US) A2.3L I/0562 (2006.01) A2.3L I/053 (2006.01) (73) Assignee: ACME SPECIALTY A2.3L I/054 (2006.01) PRODUCTS, LLC, Tampa, FL A2.3L I/0524 (2006.01) (US) A2.3L I/0522 (2006.01) A2.3L I/0532 (2006.01) (21) Appl. No.: 13/455,520 A2.3L I/0534 (2006.01) A2.3L I/0526 (2006.01) (22) Filed: Apr. 25, 2012 A23G 3/42 (2006.01) O O A63L/453 (2006.01) Related U.S. Application Data (52) U.S. Cl. ......... 424/737; 514/326; 424/769; 424/760; (60) Provisional application No. 61/480,102, filed on Apr. 424/754; 424/755; 424/756; 514/718; 514/627; 28, 2011. 424/739; 514/425; 514/548; 426/650: 426/573; 426/576; 426/577; 426/578; 426/575 Publication Classification (57) ABSTRACT (51) Int. Cl. A23G 3/36 (2006.01) Disclosed are edible formulations, for example, edible films A6 IK 36/28 (2006.01) and gummi confectioneries, that mask the taste of semen. The A6 IK 36/758 (2006.01) films and confectioneries include a composition that upon A6 IK 36/8 (2006.01) activation in the oral cavity provides a lasting taste masking A6 IK 36/8962 (2006.01) effect that endures for up to 20 minutes. -

Preparation of N Butyl Bromide Lab Report
Preparation Of N Butyl Bromide Lab Report Hoiden Wendell ingurgitate some Ellis and imperilling his odyssey so less! Embowed Preston usually flow some yeanling or readapts injudiciously. Paco is punitory and fossilizing regeneratively as miscible Phillipe inosculated intently and erased halfway. In reactivity of asking others to perform this helps facilitate the bromide lab report is reduced extensor muscle necrosis was performed Exp 23 Synthesis of n-Butyl Bromide and t-Pentyl 99-107. Organic Syntheses Procedure. Do this page is used a feasible to lighten the preparation of configuration at infinite dilution have to a sterically hindered aromatic stabilization and relative reactivity. Williamson ether synthesis video Khan Academy. The energy barrier is a haloalkane, is used a test tube. The total stereoselective synthesis of cis insect sex attractants. Experiment you will raid a Grignard reagent and react it incorporate an ester to near a tertiary alcohol Specifically in this reaction you also prepare phenyl magnesium bromide from. The boiling point of n-butyl bromide is 100 oC while expression of n-butyl chloride is 77. Substitution-lab Metabolomics. Some properties in this technique with an iron in after each reaction is added tantalum or primary halides. The records will be needed to generate lab reports at some point remains the. In today's experiment you will carry out the first gear of the barbiturate synthesis In reactions involving. Reactions of Tertiary Phosphites with Alkyl Iodides in Acetonitrile. Experiment will just the SN reactivity of alkyl halides To some worry this experiment is trap to the. Discussed here react more rapidly in solutions prepared from these solvents. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Equilibrium Structures and Vibrational Assignments for Isoamyl Alcohol and Tert-Amyl Alcohol: a Density Functional Study
Equilibrium Structures and Vibrational Assignments for Isoamyl Alcohol and tert-Amyl Alcohol: A Density Functional Study Wolfgang Forner¨ and Hassan M. Badawi Department of Chemistry, King Fahd University of Petroleum and Minerals, Dhahran 31261, Saudi Arabia Reprint requests to Wolfgang Forner.¨ E-mail: [email protected] Z. Naturforsch. 2013, 68b, 841 – 851 / DOI: 10.5560/ZNB.2013-3003 Received February 9, 2013 We have calculated the vibrational spectra of isoamyl alcohol and of tert-amyl alcohol using Den- sity Functional Theory (DFT) with the Becke-3 Lee Yang Parr (B3LYP) functional and a 6-311+G** atomic basis set. The energies of the conformers were also calculated with ab initio Perturbation Theory of second (MP2) and fourth order restricted to single, double and quadruple excitations (MP4 SDQ) with the same basis set. We found rather complicated equilibria of four conformations in each case, counting only those with appreciable abundancies. PED data were compared with GAUSSVIEW animations. The calculated wavenumbers agree rather well with the experimental ones when the gauche-trans conformer is assumed as the most important one for isoamyl alcohol, and the gauche- gauche one for tert-amyl alcohol. However, some of the experimental bands had to be assigned also to other conformers, indicating their presence in the equilibrium mixture. Due to sterical reasons both the CO and the OH bonds appear to be weaker in the tertiary alcohol, considering the wavenumbers of the CO and OH bond stretching vibrations. The bond lengths point into the same direction, however, the OH bond in the tertiary alcohol is only slightly longer than that in the primary alcohol. -

TERTIARY ACETYLENIC ALCOHOLS Compound, Ethchlorvynol (S 94) Is
36 H. ISBELL & T. L. CHRUSCIEL TERTIARY ACETYLENIC ALCOHOLS Only two compounds are listed (Table IV). Of 152. Csarza-Perez, J., Lal, S. & Lopez, E. (1967) Med. these, methylpentynol (S 95) is a weak, short-acting Serv. J. Can., 23, No. 5, 775-778 (Addiction to hypnotic. Sales have been low and few instances chlorvynol) of abuse have been reported.155' 157 The other 153. Essig, C. F. (1964) Clin. Pharmacol., 5, 334 (Addic- compound, ethchlorvynol (S 94) is more potent and tion to sedatives and tranquilizers) instances of abuse are more frequent. There is 154. Government of the United States of America, stiong clinical documentation for abrupt withdrawal US Food and Drug Administration (1965) Back- of ethchlorvynol being followed by convulsions and ground material supplied to Advisory Committee delirium.151-154, 156 Accordingly, ethchlorvynol must on Abuse of Stimulant and Depressant Drugs, be judged to have moderate abuse potential and 27 December 1965, Washington, D.C. 155. Hitsche, B. & Herbst, A. (1967) Psychiat. et methylpentynol must, by analogy, be regarded as Neurol. (Basel), 153, 308-318 (Beobachtungen having dependence potential equivalent to that of bei Missbrauch von Methylpentinol) ethchlorvynol. 156. Hudson, H. S. & Walker, H. I. (1961) Amer. J. Psychiat., 118, 361 (Withdrawal symptoms REFERENCES following ethchlorvynol (Placidyl) dependence) 157. Marley, E. & Bartholomew, A. A. (1958) J. Neurol. 151. Cohn, C. H. (1959) Canad. med. Ass. J., 81, 733 Neurosurg. Psychiat., 21, 129-140 (Clinical (Intoxication by ethchlorvynol-Placidyl) aspects of susceptibility of methylpentol) CYCLIC ETHERS The only compound in this group is paraldehyde dogs physically dependent on barbital. -

(12) United States Patent (10) Patent No.: US 8.449,908 B2 Stinchcomb Et Al
USOO84499.08B2 (12) United States Patent (10) Patent No.: US 8.449,908 B2 Stinchcomb et al. (45) Date of Patent: May 28, 2013 (54) TRANSDERMAL DELIVERY OF 4,663,474 A 5, 1987 Urban CANNABIDOL 4,876,276 A 10, 1989 Mechoulam et al. 5,059,426 A * 10/1991 Chiang et al. ................. 424/449 5,223,262 A 6/1993 Kim et al. (75) Inventors: Audra L. Stinchcomb, Lexington, KY 5,254,346 A 10, 1993 Tucker et al. (US); Buchi N. Nalluri, Lexington, KY (US) (Continued) FOREIGN PATENT DOCUMENTS (73) Assignee: Alltranz, LLC, Lexington, KY (US) EP 0570920 A1 5, 1992 EP O656354 B1 12/1993 (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 (Continued) U.S.C. 154(b) by 1546 days. OTHER PUBLICATIONS (21) Appl. No.: 11/157,034 "Cannabinoid system as a potential target for drug development in the treatment of cardiovascular disease” by Mendizabal et al., Current (22) Filed: Jun. 20, 2005 Vascular Pharmacology, 2003, vol. 1, No. 3, 301-313.* (65) Prior Publication Data (Continued) US 2005/0266.061 A1 Dec. 1, 2005 Primary Examiner — Isis Ghali (74) Attorney, Agent, or Firm — Foley & Lardner LLP (51) Int. C. A6DF 3/00 (2006.01) (57) ABSTRACT A6DF 3/02 (2006.01) A 6LX 9/70 (2006.01) The present invention overcomes the problems associated A6IL 15/16 (2006.01) with existing drug delivery systems by delivering cannab U.S. C. inoids transdermally. Preferably, the cannabinoids are deliv (52) ered via an occlusive body (i.e., a patch) to alleviate harmful USPC ...........................