Summary of Product Characteristics 1. Name of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Product Characteristics
Proposed var 24 psusa cilazapril SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [Fosinopril sodium 10 mg, tablets] [Fosinopril sodium 20 mg, tablets] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 10 or 20 mg fosinopril sodium. Excipient with known effect: Each tablet fosinopril sodium 10 mg contains 87 mg of lactose, anhydrous. Each tablet fosinopril sodium 20 mg contains 174 mg of lactose, anhydrous. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. The 10 mg tablets are white and shaped like a capsule with indents. On one side they are engraved with the letters “APO” and on the other side with “FOS-10”. The 20 mg tablets are white and their shape is oval. On one side they are engraved with the letters “APO” and on the other side with “FOS-20”. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Treatment of hypertension. - Treatment of symptomatic heart failure. 4.2 Posology and method of administration Posology Fosinopril sodium should be administered orally in a single daily dose. As with all other medicinal products taken once daily, it should be taken at approximately the same time each day. The absorption of fosinopril sodium is not affected by food. The dose should be individualised according to patient profile and blood pressure response (see section 4.4). Hypertension: Fosinopril sodium may be used as a monotherapy or in combination with other classes of antihypertensive medicinal products (see section 4.3, 4.4, 4.5 and 5.1). Hypertensive patients not being treated with diuretics: Starting dose The initial recommended dose is 10 mg once a day. -

Summary of Product Characteristics 1. Name Of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ramipril/Amlodipine Glenmark, 5 mg/5 mg, hard capsules Ramipril/Amlodipine Glenmark, 5 mg/10 mg, hard capsules Ramipril/Amlodipine Glenmark, 10 mg/5 mg, hard capsules Ramipril/Amlodipine Glenmark, 10 mg/10 mg, hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Ramipril/Amlodipine Glenmark, 5 mg/5 mg, hard capsules: each capsule contains 5 mg ramipril and amlodipine besilate equivalent to 5 mg amlodipine. Ramipril/Amlodipine Glenmark, 5 mg/10 mg, hard capsules: each capsule contains 5 mg ramipril and amlodipine besilate equivalent to 10 mg amlodipine and . Ramipril/Amlodipine Glenmark, 10 mg/5 mg, hard capsules: each capsule contains 10 mg ramipril and amlodipine besilate equivalent to5 mg amlodipine. Ramipril/Amlodipine Glenmark, 10 mg/10 mg, hard capsules: each capsule contains 10 mg ramipril and amlodipine besilate equivalent to 10 mg amlodipine . For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Hard capsule Ramipril/Amlodipine Glenmark, 5 mg/5 mg, hard capsules: hard gelatin capsules with a length of 19 mm, cap: opaque pink colour, body: opaque white colour. Content of capsules: white or almost white powder. Ramipril/Amlodipine Glenmark, 5 mg/10 mg, hard capsules: hard gelatin capsules with a length of 19 mm, cap: opaque red - brown colour, body: opaque white colour. Content of capsules: white or almost white powder. Ramipril/Amlodipine Glenmark, 10 mg/5 mg, hard capsules: hard gelatin capsules with a length of 19 mm, cap: opaque dark pink colour, body: opaque white colour. Content of capsules: white or almost white powder. -

Medication Risks in Older Patients with Cancer
Medication risks in older patients with cancer 1 Medication risks in older patients (70+) with cancer and their association with therapy-related toxicity Imke Ortland1, Monique Mendel Ott1, Michael Kowar2, Christoph Sippel3, Yon-Dschun Ko3#, Andreas H. Jacobs2#, Ulrich Jaehde1# 1 Institute of Pharmacy, Department of Clinical Pharmacy, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany 2 Department of Geriatrics and Neurology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany 3 Department of Oncology and Hematology, Johanniter Hospital Bonn, Johanniterstr. 1-3, 53113 Bonn, Germany # equal contribution Corresponding author Ulrich Jaehde Institute of Pharmacy University of Bonn An der Immenburg 4 53121 Bonn, Germany Phone: +49 228-73-5252 Fax: +49-228-73-9757 [email protected] Medication risks in older patients with cancer 2 Abstract Objectives To evaluate medication-related risks in older patients with cancer and their association with severe toxicity during antineoplastic therapy. Methods This is a secondary analysis of two prospective, single-center observational studies which included patients ≥ 70 years with cancer. The patients’ medication was investigated regarding possible risks: polymedication (defined as the use of ≥ 5 drugs), potentially inadequate medication (PIM; defined by the EU(7)-PIM list), and relevant potential drug- drug interactions (rPDDI; analyzed by the ABDA interaction database). The risks were analyzed at two different time points: before and after start of cancer therapy. Severe toxicity during antineoplastic therapy was captured from medical records according to the Common Terminology Criteria for Adverse Events (CTCAE). The association between Grade ≥ 3 toxicity and medication risks was evaluated by univariate regression. -

Summary of Product Characteristics 1. NAME of the MEDICINAL
Summary of product characteristics 1. NAME OF THE MEDICINAL PRODUCT Losartan Potassium 50 mg Film-coated Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 50 mg of losartan potassium, equivalent to 45.8mg of Losartan. Excipient: 52mg of lactose/film-coated tablet. For the full list of excipients see section 6.1 3. PHARMACEUTICAL FORM Film-coated tablet White to off white, round, biconvex, film-coated tablets with breakline on one side and “50” debossing on other side. The break line is only to facilitate breaking for ease of swallowing and not to divide into equal doses 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of essential hypertension in adults and in children and adolescents 6-18 years of age. Treatment of renal disease in adult patients with hypertension and type 2 diabetes mellitus with proteinuria 0.5 g/day as part of an antihypertensive treatment (see sections 4.3, 4.4, 4.5 and 5.1). Treatment of chronic heart failure in adult patients when treatment with Angiotensin converting enzyme (ACE) inhibitors is not considered suitable due to incompatibility, especially cough, or contraindication. Patients with heart failure who have been stabilised with an ACE inhibitor should not be switched to losartan. The patients should have a left ventricular ejection fraction ≤ 40% and should be clinically stable and on an established treatment regimen for chronic heart failure. Reduction in the risk of stroke in adult hypertensive patients with left ventricular hypertrophy documented by ECG (see section 5.1 LIFE study, Race). 4.2 Posology and method of administration Posology Hypertension The usual starting and maintenance dose is 50 mg once daily for most patients. -

COZAAR (Losartan Potassium) Tablets, for Oral Use
HIGHLIGHTS OF PRESCRIBING INFORMATION • Increase dose to 100 mg once daily if further blood pressure These highlights do not include all the information needed to use response is needed. (2.3) COZAAR safely and effectively. See full prescribing information for COZAAR. --------------------- DOSAGE FORMS AND STRENGTHS --------------------- Tablets: 25 mg; 50 mg; and 100 mg. (3) ® COZAAR (losartan potassium) tablets, for oral use ------------------------------- CONTRAINDICATIONS ------------------------------- Initial U.S. Approval: 1995 • Hypersensitivity to any component. (4) • Coadministration with aliskiren in patients with diabetes. (4) WARNING: FETAL TOXICITY See full prescribing information for complete boxed warning. ----------------------- WARNINGS AND PRECAUTIONS ----------------------- • Hypotension: Correct volume or salt depletion prior to administration When pregnancy is detected, discontinue COZAAR as soon as of COZAAR. (5.2) possible. Drugs that act directly on the renin-angiotensin system • Monitor renal function and potassium in susceptible patients. (5.3, can cause injury and death to the developing fetus. (5.1) 5.4) --------------------------- RECENT MAJOR CHANGES --------------------------- ------------------------------ ADVERSE REACTIONS ------------------------------ Warnings and Precautions Hyperkalemia (5.4) 10/2018 Most common adverse reactions (incidence ≥2% and greater than placebo) are: dizziness, upper respiratory infection, nasal congestion, ----------------------------INDICATIONS AND USAGE ---------------------------- and back pain. (6.1) COZAAR is an angiotensin II receptor blocker (ARB) indicated for: • Treatment of hypertension, to lower blood pressure in adults and To report SUSPECTED ADVERSE REACTIONS, contact Merck children greater than 6 years old. Lowering blood pressure reduces Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877- the risk of fatal and nonfatal cardiovascular events, primarily strokes 888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -

COZAAR® (LOSARTAN POTASSIUM TABLETS) Page 1 of 28
COZAAR® (LOSARTAN POTASSIUM TABLETS) Page 1 of 28 COZAAR® (LOSARTAN POTASSIUM TABLETS) USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, COZAAR® should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality. DESCRIPTION COZAAR (losartan potassium) is an angiotensin II receptor (type AT1) antagonist. Losartan potassium, a non- peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole- 5-methanol monopotassium salt. Its empirical formula is C22H22ClKN6O, and its structural formula is: Losartan potassium is a white to off-white free-flowing crystalline powder with a molecular weight of 461.01. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone. Oxidation of the 5-hydroxymethyl group on the imidazole ring results in the active metabolite of losartan. COZAAR is available as tablets for oral administration containing either 25 mg, 50 mg or 100 mg of losartan potassium and the following inactive ingredients: microcrystalline cellulose, lactose hydrous, pregelatinized starch, magnesium stearate, hydroxypropyl cellulose, hypromellose, and titanium dioxide. COZAAR 25 mg, 50 mg and 100 mg tablets contain potassium in the following amounts: 2.12 mg (0.054 mEq), 4.24 mg (0.108 mEq) and 8.48 mg (0.216 mEq), respectively. COZAAR 25 mg, COZAAR 50 mg, and COZAAR 100 mg may also contain carnauba wax. -

Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin
DOI: 10.7860/JCDR/2018/31983.11754 Original Article Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin Pharmacology Section Receptor Blockers-A Pilot Study S BHUVANESHWARI1, PRAKASH VEL SANKHYA SAROJ2, D VIJAYA3, M SABARI SOWMYA4, R SENTHIL KUMAR5 ABSTRACT Results: Among all, 48% (24 out of 50) of the study population Introduction: Hyponatremia, serum sodium <135 mmol/L, can administered with ACEI and ARB developed hyponatremia. result in neurological manifestation in acute cases, may lead Predisposition to develop hyponatremia was high in males to seizures and coma. Angiotensin Converting Enzyme Inhibitor compared to females. Incidence of hyponatremia was 62.5% (10 (ACEI) and Angiotensin II Receptor Blockers (ARB) are drugs out of 16) in the age group of 56-75 years. Though, incidence of that have been commonly prescribed for the treatment of hyponatremia was 54.5% (18 out of 33) in ACEI group compared hypertension and cardiac diseases. It has become important to 35.2% (6 out of 17) in ARB group, but it was not statistically to evaluate and investigate the incidence of hyponatremia on significant. The study also revealed that metosartan had a higher consumption of these drugs. association with hyponatremia compared to other drugs. Aim: To determine the susceptibility of patients on ACEI and Conclusion: Hyponatremia was induced in nearly 50% of ARB to hyponatremia and to ascertain the drug producing patients taking ACEI and ARB. The incidence of hyponatremia notable hyponatremia among ACEI and ARB. among patients on these two drugs did not show statistical variation. Metosartan showed a higher incidence of Materials and Methods: The study was conducted in a tertiary hyponatremia compared to enalapril, ramipril, captopril, care hospital. -

Telmisartan Vs Losartan Plus Hydrochlorothiazide in the Treatment of Mild-To-Moderate Essential Hypertensionfa Randomised ABPM Study
Journal of Human Hypertension (2003) 17, 569–575 & 2003 Nature Publishing Group All rights reserved 0950-9240/03 $25.00 www.nature.com/jhh ORIGINAL ARTICLE Telmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertensionFa randomised ABPM study JM Neutel1 , RE Kolloch2, PF Plouin3, TW Meinicke4 and H Schumacher5 for the OTELLOH Study Group 1Orange County Heart Institute & Research Center, Orange, CA, USA; 2Krankenanstalten Gilead, Bielefeld, Germany; 3Hoˆpital Broussais, Paris, France; 4Clinical Research Boehringer Ingelheim Pharma KG, Biberach, Germany; 5Medical Data Services Boehringer Ingelheim Pharma KG, Ingelheim, Germany The objective of this prospective, randomised, open- null hypothesis of a treatment difference of 43 mmHg. label, blinded-end point parallel-group, multicentre The reduction in mean 24-h systolic blood pressure was study was to show that telmisartan 80 mg is not inferior 13.2 7 10.2 mmHg with telmisartan and 17.1 7 10.3 to a fixed-dose combination of losartan 50 mg/hydro- mmHg with losartan/HCTZ. Both drugs provided effec- chlorothiazide (HCTZ) 12.5 mg in patients with mild-to- tive control over the 24-h dosing interval. Analyses of moderate hypertension. The criterion for noninferiority morning (0600–1159) ambulatory blood pressure mon- was a treatment difference of p3.0 mmHg in the reduc- itoring DBP means and trough cuff DBP confirmed the tion of 24-h mean ambulatory diastolic blood pressure noninferiority hypothesis of the protocol for telmisartan (DBP) from the end of the 4-week placebo washout 80 mg vs losartan 50 mg/HCTZ 12.5 mg. The reductions period to the end of the 6-week active treatment period. -

Part1 Gendiff.Qxp
Sex Differences in Health Status, Health Care Use, and Quality of Care: A Population-Based Analysis for Manitoba’s Regional Health Authorities November 2005 Manitoba Centre for Health Policy Department of Community Health Sciences Faculty of Medicine, University of Manitoba Randy Fransoo, MSc Patricia Martens, PhD The Need to KnowTeam (funded through CIHR) Elaine Burland, MSc Heather Prior, MSc Charles Burchill, MSc Dan Chateau, PhD Randy Walld, BSc, BComm (Hons) This report is produced and published by the Manitoba Centre for Health Policy (MCHP). It is also available in PDF format on our website at http://www.umanitoba.ca/centres/mchp/reports.htm Information concerning this report or any other report produced by MCHP can be obtained by contacting: Manitoba Centre for Health Policy Dept. of Community Health Sciences Faculty of Medicine, University of Manitoba 4th Floor, Room 408 727 McDermot Avenue Winnipeg, Manitoba, Canada R3E 3P5 Email: [email protected] Order line: (204) 789 3805 Reception: (204) 789 3819 Fax: (204) 789 3910 How to cite this report: Fransoo R, Martens P, The Need To Know Team (funded through CIHR), Burland E, Prior H, Burchill C, Chateau D, Walld R. Sex Differences in Health Status, Health Care Use and Quality of Care: A Population-Based Analysis for Manitoba’s Regional Health Authorities. Winnipeg, Manitoba Centre for Health Policy, November 2005. Legal Deposit: Manitoba Legislative Library National Library of Canada ISBN 1-896489-20-6 ©Manitoba Health This report may be reproduced, in whole or in part, provided the source is cited. 1st Printing 10/27/2005 THE MANITOBA CENTRE FOR HEALTH POLICY The Manitoba Centre for Health Policy (MCHP) is located within the Department of Community Health Sciences, Faculty of Medicine, University of Manitoba. -
![5 Mg Film-Coated Tablets [Nationally Completed Name] 10 Mg Film-Coated Tablets [Nationally Completed Name] 20 Mg Film-Coated Tablets](https://docslib.b-cdn.net/cover/3029/5-mg-film-coated-tablets-nationally-completed-name-10-mg-film-coated-tablets-nationally-completed-name-20-mg-film-coated-tablets-1833029.webp)
5 Mg Film-Coated Tablets [Nationally Completed Name] 10 Mg Film-Coated Tablets [Nationally Completed Name] 20 Mg Film-Coated Tablets
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [nationally completed name] 5 mg film-coated tablets [nationally completed name] 10 mg film-coated tablets [nationally completed name] 20 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains 5/10/20 mg Benazepril hydrochloride. For the full list of excipients see section 6.1 3. PHARMACEUTICAL FORM Film-coated tablet [nationally completed name] 5 mg film-coated tablets: Oval (4 x 8 mm), light yellow film-coated tablets with score on both sides. [nationally completed name] 10 mg film-coated tablets: Oval (11 x 5.5 mm) yellow film-coated tablets with score on both sides. [nationally completed name] 20 mg film-coated tablets: Oval (11 x 5.5 mm) light red film-coated tablet with score on both sides. The tablet can be divided into equal doses. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Essential hypertension, congestive heart failure - in addition to diuretics and especially to digitalis glycosides in cases of severe congestive heart failure 4.2 Posology and method of administration Posology Paediatric population [nationally completed name] is not recommended for use in children due to insufficient data on safety and efficacy (see section 4.4). Essential hypertension 10 to 20 mg daily divided in one or two doses. Maximum daily dose is 40 mg. Congestive heart failure Initially 2.5 mg daily. After 2-4 weeks the dosage can be increased to 5 mg daily. Maximum dose is 20 mg daily. Dosage at renal impairment For the treatment of essential hypertension the dose should be reduced if creatinine clearance is below 30 ml/min. -

Chapter 2 Methodological Aspects
Chapter 2 Methodological aspects Chapter 2.1 Indications for antihypertensive drug use in a prescription database BLG van Wijk, OH Klungel, ER Heerdink, A de Boer Department of Pharmacoepidemiology & Pharmacotherapy, Utrecht Institute for Pharmaceutical Sciences, Utrecht University Submitted CHAPTER 2.1 Summary Background: Drugs termed antihypertensives are prescribed and registered for other indications than hypertension alone. In pharmacoepidemiological studies, this could introduce several forms of bias. The objective of this study was to determine the extent to which drugs classified as antihypertensive drugs are prescribed for other diseases than hypertension. Methods: The NIVEL database was used, containing prescriptions from a sample of general practitioners in The Netherlands. Classes of antihypertensive drugs were analyzed separately, based on ATC-codes: miscellaneous antihypertensives, diuretics, beta-blockers, calcium channel blockers and agents acting on the renin angiotensin system. In addition, these classes were further subdivided based on their specific mechanism of action. All first prescriptions of a patient in the database for an antihypertensive drug were selected. ICPC diagnoses studied were: increased blood pressure, hypertension without organ damage, hypertension with organ damage and hypertension with diabetes mellitus for which the above- defined antihypertensive drugs were prescribed. Results: Of 24,812 patients who received a first prescription for an antihypertensive drug, 63.0% received a first prescription for hypertension related diagnoses (diuretics: 54.1%, beta-blockers: 59.1%, calcium channel blockers: 60.3%, agents acting on the renin angiotensin system: 82.8% and miscellaneous antihypertensives: 64.6%). Subdividing and restricting these subgroups based on their mechanism of action yields a higher percentage of first prescriptions with hypertension related diagnoses (low-ceiling diuretics: 78.8%, selective beta- blockers: 69.9% and dihydropyridine calcium channel blockers: 76.8%). -
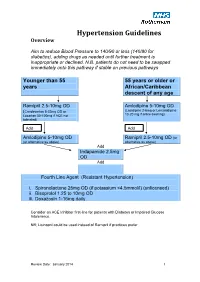
Hypertension Protocol
Hypertension Guidelines Overview Aim to reduce Blood Pressure to 140/90 or less (140/80 for diabetics), adding drugs as needed until further treatment is inappropriate or declined. N.B. patients do not need to be swapped immediately onto this pathway if stable on previous pathways Younger than 55 55 years or older or years African/Caribbean descent of any age Ramipril 2.5-10mg OD Amlodipine 5-10mg OD (Candesartan 8-32mg OD or (Lacidipine 2-6mg or Lercanidipine 10-20 mg if ankle swelling) Losartan 50-100mg if ACE not tolerated) Add Add Amlodipine 5-10mg OD Ramipril 2.5-10mg OD (or (or alternative as above) alternative as above) Add Indapamide 2.5mg OD Add Fourth Line Agent (Resistant Hypertension) i. Spironolactone 25mg OD (if potassium <4.5mmol/l) (unlicensed) ii. Bisoprolol 1.25 to 10mg OD iii. Doxazosin 1-16mg daily Consider an ACE Inhibitor first-line for patients with Diabetes or Impaired Glucose Intolerance. NB; Lisinopril could be used instead of Ramipril if practices prefer Review Date: January 2014 1 Review Date: January 2014 2 General Principles 1. Clinic BP >140/90 leads to Ambulatory Blood Pressure Monitoring (ABPM) 2. ABPM uses average of at least 14 readings taken during waking hours 3. Offer antihypertensive drug to all under 80 with Stage 1 hypertension with: o Target organ damage (high albumin:creatinine ratio, haematuria, raised creatinine, low eGFR, hypertensive retinopathy, LVH) o Established cardiovascular disease o Renal disease o Diabetes o 10 year Cardiovascular risk >20% 4. Offer antihypertensive drug to all with stage 2 hypertension 5.