Cozaar® (Losartan Potassium Tablets)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Valturna Label
HIGHLIGHTS OF PRESCRIBING INFORMATION -------------------------WARNINGS AND PRECAUTIONS---------------- These highlights do not include all the information needed to use • Avoid fetal or neonatal exposure. (5.1) Valturna safely and effectively. See full prescribing information for • Head and neck angioedema: Discontinue Valturna and monitor until Valturna. signs and symptoms resolve. (5.2) • Hypotension in volume- or salt-depleted Patients: Correct imbalances Valturna (aliskiren and valsartan, USP) Tablets before initiating therapy with Valturna. (5.3) Initial U.S. Approval: 2009 • Patients with renal impairment: Decreases in renal function may be anticipated in susceptible individuals. (5.4) WARNING: AVOID USE IN PREGNANCY • Patients with hepatic impairment: Slower clearance may occur. (5.5) See full prescribing information for complete boxed warning. • Hyperkalemia: Consider periodic determinations of serum electrolytes to When pregnancy is detected, discontinue Valturna as soon as possible. detect possible electrolyte imbalances, particularly in patients at risk. When used in pregnancy during the second and third trimester, drugs (5.7) that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. (5.1) --------------------------------ADVERSE REACTIONS----------------------- The most common adverse events (incidence ≥1.5% and more common than ---------------------------INDICATIONS AND USAGE----------------------- with placebo) are: fatigue and nasopharyngitis. (6.1) Valturna is a combination of aliskiren, a direct renin inhibitor, and valsartan, an angiotensin II receptor blocker (ARB), indicated for the treatment of To report SUSPECTED ADVERSE REACTIONS, contact Novartis hypertension: Pharmaceuticals Corporation at 1-888-669-6682 or FDA at • In patients not adequately controlled with monotherapy. (1) 1-800-FDA-1088 or www.fda.gov/medwatch • May be substituted for titrated components. -

Patient Information Telmisartan (TEL-Mi-SAR-Tan) Tablets, USP
Patient Information Telmisartan (TEL-mi-SAR-tan) Tablets, USP Read this Patient Information before you start taking telmisartan tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment. What is the most important information I should know about telmisartan tablets? Telmisartan tablets can cause harm or death to an unborn baby. Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant. If you get pregnant while taking telmisartan tablets, tell your doctor right away. What are telmisartan tablets? Telmisartan tablets are a prescription medicine used: • to treat high blood pressure (hypertension) It is not known if telmisartan tablets are safe and effective in children. Who should not take telmisartan tablets? You should not take telmisartan tablets if you are allergic (hypersensitive) to the active ingredient (telmisartan) or any of the other ingredients listed at the end of this leaflet. For patients with diabetes, if you are taking telmisartan tablets you should not take aliskiren. What should I tell my doctor before taking telmisartan tablets? Before you take telmisartan tablets, tell your doctor if you: • are pregnant or are planning to become pregnant. See “What is the most important information I should know about telmisartan tablets?” • are breast-feeding or plan to breast-feed. It is not known if telmisartan passes into your breast milk. You and your doctor should decide if you will take telmisartan tablets or breast-feed. You should not do both. -

Effect of Aldosterone Breakthrough on Albuminuria During Treatment with a Direct Renin Inhibitor and Combined Effect with a Mineralocorticoid Receptor Antagonist
Hypertension Research (2013) 36, 879–884 & 2013 The Japanese Society of Hypertension All rights reserved 0916-9636/13 www.nature.com/hr ORIGINAL ARTICLE Effect of aldosterone breakthrough on albuminuria during treatment with a direct renin inhibitor and combined effect with a mineralocorticoid receptor antagonist Atsuhisa Sato and Seiichi Fukuda We have reported observing aldosterone breakthrough in the course of relatively long-term treatment with renin–angiotensin (RA) system inhibitors, where the plasma aldosterone concentration (PAC) increased following an initial decrease. Aldosterone breakthrough has the potential to eliminate the organ-protective effects of RA system inhibitors. We therefore conducted a study in essential hypertensive patients to determine whether aldosterone breakthrough occurred during treatment with the direct renin inhibitor (DRI) aliskiren and to ascertain its clinical significance. The study included 40 essential hypertensive patients (18 men and 22 women) who had been treated for 12 months with aliskiren. Aliskiren significantly decreased blood pressure and plasma renin activity (PRA). The PAC was also decreased significantly at 3 and 6 months; however, the significant difference disappeared after 12 months. Aldosterone breakthrough was observed in 22 of the subjects (55%). Urinary albumin excretion differed depending on whether breakthrough occurred. For the subjects in whom aldosterone breakthrough was observed, eplerenone was added. A significant decrease in urinary albumin excretion was observed after 1 month, independent of changes in blood pressure. In conclusion, this study demonstrated that aldosterone breakthrough occurs in some patients undergoing DRI therapy. Aldosterone breakthrough affects the drug’s ability to improve urinary albumin excretion, and combining a mineralocorticoid receptor antagonist with the DRI may be useful for decreasing urinary albumin excretion. -

A Comparison of the Tolerability of the Direct Renin Inhibitor Aliskiren and Lisinopril in Patients with Severe Hypertension
Journal of Human Hypertension (2007) 21, 780–787 & 2007 Nature Publishing Group All rights reserved 0950-9240/07 $30.00 www.nature.com/jhh ORIGINAL ARTICLE A comparison of the tolerability of the direct renin inhibitor aliskiren and lisinopril in patients with severe hypertension RH Strasser1, JG Puig2, C Farsang3, M Croket4,JLi5 and H van Ingen4 1Technical University Dresden, Heart Center, University Hospital, Dresden, Germany; 2Department of Internal Medicine, La Paz Hospital, Madrid, Spain; 31st Department of Internal Medicine, Semmelweis University, Budapest, Hungary; 4Novartis Pharma AG, Basel, Switzerland and 5Novartis Institutes for Biomedical Research, Cambridge, MA, USA Patients with severe hypertension (4180/110 mm Hg) LIS 3.4%). The most frequently reported AEs in both require large blood pressure (BP) reductions to reach groups were headache, nasopharyngitis and dizziness. recommended treatment goals (o140/90 mm Hg) and At end point, ALI showed similar mean reductions from usually require combination therapy to do so. This baseline to LIS in msDBP (ALI À18.5 mm Hg vs LIS 8-week, multicenter, randomized, double-blind, parallel- À20.1 mm Hg; mean treatment difference 1.7 mm Hg group study compared the tolerability and antihyperten- (95% confidence interval (CI) À1.0, 4.4)) and mean sitting sive efficacy of the novel direct renin inhibitor aliskiren systolic blood pressure (ALI À20.0 mm Hg vs LIS with the angiotensin converting enzyme inhibitor À22.3 mm Hg; mean treatment difference 2.8 mm Hg lisinopril in patients with severe hypertension (mean (95% CI À1.7, 7.4)). Responder rates (msDBPo90 mm Hg sitting diastolic blood pressure (msDBP)X105 mm Hg and/or reduction from baselineX10 mm Hg) were 81.5% and o120 mm Hg). -

Summary of Product Characteristics
Proposed var 24 psusa cilazapril SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT [Fosinopril sodium 10 mg, tablets] [Fosinopril sodium 20 mg, tablets] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 10 or 20 mg fosinopril sodium. Excipient with known effect: Each tablet fosinopril sodium 10 mg contains 87 mg of lactose, anhydrous. Each tablet fosinopril sodium 20 mg contains 174 mg of lactose, anhydrous. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. The 10 mg tablets are white and shaped like a capsule with indents. On one side they are engraved with the letters “APO” and on the other side with “FOS-10”. The 20 mg tablets are white and their shape is oval. On one side they are engraved with the letters “APO” and on the other side with “FOS-20”. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications - Treatment of hypertension. - Treatment of symptomatic heart failure. 4.2 Posology and method of administration Posology Fosinopril sodium should be administered orally in a single daily dose. As with all other medicinal products taken once daily, it should be taken at approximately the same time each day. The absorption of fosinopril sodium is not affected by food. The dose should be individualised according to patient profile and blood pressure response (see section 4.4). Hypertension: Fosinopril sodium may be used as a monotherapy or in combination with other classes of antihypertensive medicinal products (see section 4.3, 4.4, 4.5 and 5.1). Hypertensive patients not being treated with diuretics: Starting dose The initial recommended dose is 10 mg once a day. -

COZAAR (Losartan Potassium) Tablets, for Oral Use
HIGHLIGHTS OF PRESCRIBING INFORMATION • Increase dose to 100 mg once daily if further blood pressure These highlights do not include all the information needed to use response is needed. (2.3) COZAAR safely and effectively. See full prescribing information for COZAAR. --------------------- DOSAGE FORMS AND STRENGTHS --------------------- Tablets: 25 mg; 50 mg; and 100 mg. (3) ® COZAAR (losartan potassium) tablets, for oral use ------------------------------- CONTRAINDICATIONS ------------------------------- Initial U.S. Approval: 1995 • Hypersensitivity to any component. (4) • Coadministration with aliskiren in patients with diabetes. (4) WARNING: FETAL TOXICITY See full prescribing information for complete boxed warning. ----------------------- WARNINGS AND PRECAUTIONS ----------------------- • Hypotension: Correct volume or salt depletion prior to administration When pregnancy is detected, discontinue COZAAR as soon as of COZAAR. (5.2) possible. Drugs that act directly on the renin-angiotensin system • Monitor renal function and potassium in susceptible patients. (5.3, can cause injury and death to the developing fetus. (5.1) 5.4) --------------------------- RECENT MAJOR CHANGES --------------------------- ------------------------------ ADVERSE REACTIONS ------------------------------ Warnings and Precautions Hyperkalemia (5.4) 10/2018 Most common adverse reactions (incidence ≥2% and greater than placebo) are: dizziness, upper respiratory infection, nasal congestion, ----------------------------INDICATIONS AND USAGE ---------------------------- and back pain. (6.1) COZAAR is an angiotensin II receptor blocker (ARB) indicated for: • Treatment of hypertension, to lower blood pressure in adults and To report SUSPECTED ADVERSE REACTIONS, contact Merck children greater than 6 years old. Lowering blood pressure reduces Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877- the risk of fatal and nonfatal cardiovascular events, primarily strokes 888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -

COZAAR® (LOSARTAN POTASSIUM TABLETS) Page 1 of 28
COZAAR® (LOSARTAN POTASSIUM TABLETS) Page 1 of 28 COZAAR® (LOSARTAN POTASSIUM TABLETS) USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, COZAAR® should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality. DESCRIPTION COZAAR (losartan potassium) is an angiotensin II receptor (type AT1) antagonist. Losartan potassium, a non- peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole- 5-methanol monopotassium salt. Its empirical formula is C22H22ClKN6O, and its structural formula is: Losartan potassium is a white to off-white free-flowing crystalline powder with a molecular weight of 461.01. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone. Oxidation of the 5-hydroxymethyl group on the imidazole ring results in the active metabolite of losartan. COZAAR is available as tablets for oral administration containing either 25 mg, 50 mg or 100 mg of losartan potassium and the following inactive ingredients: microcrystalline cellulose, lactose hydrous, pregelatinized starch, magnesium stearate, hydroxypropyl cellulose, hypromellose, and titanium dioxide. COZAAR 25 mg, 50 mg and 100 mg tablets contain potassium in the following amounts: 2.12 mg (0.054 mEq), 4.24 mg (0.108 mEq) and 8.48 mg (0.216 mEq), respectively. COZAAR 25 mg, COZAAR 50 mg, and COZAAR 100 mg may also contain carnauba wax. -

Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin
DOI: 10.7860/JCDR/2018/31983.11754 Original Article Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin Pharmacology Section Receptor Blockers-A Pilot Study S BHUVANESHWARI1, PRAKASH VEL SANKHYA SAROJ2, D VIJAYA3, M SABARI SOWMYA4, R SENTHIL KUMAR5 ABSTRACT Results: Among all, 48% (24 out of 50) of the study population Introduction: Hyponatremia, serum sodium <135 mmol/L, can administered with ACEI and ARB developed hyponatremia. result in neurological manifestation in acute cases, may lead Predisposition to develop hyponatremia was high in males to seizures and coma. Angiotensin Converting Enzyme Inhibitor compared to females. Incidence of hyponatremia was 62.5% (10 (ACEI) and Angiotensin II Receptor Blockers (ARB) are drugs out of 16) in the age group of 56-75 years. Though, incidence of that have been commonly prescribed for the treatment of hyponatremia was 54.5% (18 out of 33) in ACEI group compared hypertension and cardiac diseases. It has become important to 35.2% (6 out of 17) in ARB group, but it was not statistically to evaluate and investigate the incidence of hyponatremia on significant. The study also revealed that metosartan had a higher consumption of these drugs. association with hyponatremia compared to other drugs. Aim: To determine the susceptibility of patients on ACEI and Conclusion: Hyponatremia was induced in nearly 50% of ARB to hyponatremia and to ascertain the drug producing patients taking ACEI and ARB. The incidence of hyponatremia notable hyponatremia among ACEI and ARB. among patients on these two drugs did not show statistical variation. Metosartan showed a higher incidence of Materials and Methods: The study was conducted in a tertiary hyponatremia compared to enalapril, ramipril, captopril, care hospital. -

Telmisartan Vs Losartan Plus Hydrochlorothiazide in the Treatment of Mild-To-Moderate Essential Hypertensionfa Randomised ABPM Study
Journal of Human Hypertension (2003) 17, 569–575 & 2003 Nature Publishing Group All rights reserved 0950-9240/03 $25.00 www.nature.com/jhh ORIGINAL ARTICLE Telmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertensionFa randomised ABPM study JM Neutel1 , RE Kolloch2, PF Plouin3, TW Meinicke4 and H Schumacher5 for the OTELLOH Study Group 1Orange County Heart Institute & Research Center, Orange, CA, USA; 2Krankenanstalten Gilead, Bielefeld, Germany; 3Hoˆpital Broussais, Paris, France; 4Clinical Research Boehringer Ingelheim Pharma KG, Biberach, Germany; 5Medical Data Services Boehringer Ingelheim Pharma KG, Ingelheim, Germany The objective of this prospective, randomised, open- null hypothesis of a treatment difference of 43 mmHg. label, blinded-end point parallel-group, multicentre The reduction in mean 24-h systolic blood pressure was study was to show that telmisartan 80 mg is not inferior 13.2 7 10.2 mmHg with telmisartan and 17.1 7 10.3 to a fixed-dose combination of losartan 50 mg/hydro- mmHg with losartan/HCTZ. Both drugs provided effec- chlorothiazide (HCTZ) 12.5 mg in patients with mild-to- tive control over the 24-h dosing interval. Analyses of moderate hypertension. The criterion for noninferiority morning (0600–1159) ambulatory blood pressure mon- was a treatment difference of p3.0 mmHg in the reduc- itoring DBP means and trough cuff DBP confirmed the tion of 24-h mean ambulatory diastolic blood pressure noninferiority hypothesis of the protocol for telmisartan (DBP) from the end of the 4-week placebo washout 80 mg vs losartan 50 mg/HCTZ 12.5 mg. The reductions period to the end of the 6-week active treatment period. -
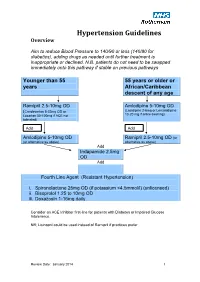
Hypertension Protocol
Hypertension Guidelines Overview Aim to reduce Blood Pressure to 140/90 or less (140/80 for diabetics), adding drugs as needed until further treatment is inappropriate or declined. N.B. patients do not need to be swapped immediately onto this pathway if stable on previous pathways Younger than 55 55 years or older or years African/Caribbean descent of any age Ramipril 2.5-10mg OD Amlodipine 5-10mg OD (Candesartan 8-32mg OD or (Lacidipine 2-6mg or Lercanidipine 10-20 mg if ankle swelling) Losartan 50-100mg if ACE not tolerated) Add Add Amlodipine 5-10mg OD Ramipril 2.5-10mg OD (or (or alternative as above) alternative as above) Add Indapamide 2.5mg OD Add Fourth Line Agent (Resistant Hypertension) i. Spironolactone 25mg OD (if potassium <4.5mmol/l) (unlicensed) ii. Bisoprolol 1.25 to 10mg OD iii. Doxazosin 1-16mg daily Consider an ACE Inhibitor first-line for patients with Diabetes or Impaired Glucose Intolerance. NB; Lisinopril could be used instead of Ramipril if practices prefer Review Date: January 2014 1 Review Date: January 2014 2 General Principles 1. Clinic BP >140/90 leads to Ambulatory Blood Pressure Monitoring (ABPM) 2. ABPM uses average of at least 14 readings taken during waking hours 3. Offer antihypertensive drug to all under 80 with Stage 1 hypertension with: o Target organ damage (high albumin:creatinine ratio, haematuria, raised creatinine, low eGFR, hypertensive retinopathy, LVH) o Established cardiovascular disease o Renal disease o Diabetes o 10 year Cardiovascular risk >20% 4. Offer antihypertensive drug to all with stage 2 hypertension 5. -

AT1-Receptor Blockers: Differences That Matter
Journal of Human Hypertension (2002) 16, S9–S16 2002 Nature Publishing Group All rights reserved 0950-9240/02 $25.00 www.nature.com/jhh AT1-receptor blockers: differences that matter AH Gradman Division of Cardiovascular Diseases, The Western Pennsylvania Hospital, Pittsburgh, Pennsylvania, USA The available angiotensin II type 1 (AT1)-receptor block- effect, and this finding is supported by the results of ers differ markedly in their pharmacological properties comparative clinical trials. Moreover, the prolonged and clinical efficacy. Losartan shifts the dose-response binding of candesartan to the receptor is reflected in a curve for angiotensin II to the right without affecting the longer duration of action, compared with losartan; the maximal response; this antagonism can be overcome by antihypertensive effect of candesartan persists for 48 h increasing concentrations of angiotensin II and thus los- after dosing, compared with approximately 24 h with artan acts as a surmountable antagonist. By contrast, losartan. Candesartan thus offers extended therapeutic other agents suppress the maximal response to angio- coverage, an important consideration since a majority tensin II to varying extents; this can not be overcome by of patients miss occasional doses of antihypertensive increasing angiotensin concentrations and hence these medication. There is currently no evidence that differ- agents are insurmountable antagonists. Receptor bind- ences in receptor binding between AT1-receptor block- ing studies have shown that candesartan has the high- ers translate into differences in tolerability. In summary, est affinity for the AT1-receptor, followed by irbesartan, therefore, pharmacological differences between AT1- valsartan and losartan, and that candesartan dis- receptor blockers are reflected in clinically important sociates from the receptor more slowly than other differences in maximal antihypertensive effect, antagonists. -

Therapeutic Class Overview Angiotensin II Receptor Blockers (Arbs) – Combination Products
Therapeutic Class Overview Angiotensin II receptor blockers (ARBs) – combination products Therapeutic Class • Overview/Summary: This review will focus on the angiotensin II receptor blocker (ARB) combination products.1-13 The renin-angiotensin-aldosterone system (RAAS) is the most important component in the homeostatic regulation of blood pressure.14,15 Excessive activity of the RAAS may lead to hypertension and disorders of fluid and electrolyte imbalance.16 Renin catalyzes the conversion of angiotensinogen to angiotensin I. Angiotensin I is then cleaved to angiotensin II by angiotensin converting enzyme (ACE). Angiotensin II can increase blood pressure by direct vasoconstriction and through actions on the brain and autonomic nervous system.14,16 In addition, angiotensin II stimulates aldosterone synthesis from the adrenal cortex, leading to sodium and water reabsorption. Angiotensin II exerts other detrimental cardiovascular effects including hypertrophy and remodeling.14,15 The RAAS plays an important role in the development and progression of heart failure.15 ACE inhibitors block the conversion of angiotensin I to angiotensin II, and also inhibit the breakdown of bradykinin, a potent vasodilator associated with dry cough.14-17 Since angiotensin II may also be generated through other pathways that do not depend upon ACE (e.g., chymase), blockade of angiotensin II by ACE inhibitors is incomplete.14,15 The ARBs block the angiotensin II receptor subtype AT1, preventing the negative effects of angiotensin II, regardless of its origin. ARBs do not appear to affect bradykinin. Amlodipine, a nondihydropyridine calcium channel blocker, inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Cardiac and vascular smooth muscle contraction depends on the movement of extracellular calcium ions into cells through specific ion channels.