Therapeutic Class Overview Angiotensin II Receptor Blockers (Arbs) – Combination Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AVAPRO Rx Only (Irbesartan) Tablets
NDA 20-757/S-038 Page 3 ® AVAPRO Rx only (irbesartan) Tablets USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, AVAPRO should be discontinued as soon as possible. See WARNINGS: Fetal/Neonatal Morbidity and Mortality. DESCRIPTION ®* AVAPRO (irbesartan) is an angiotensin II receptor (AT1 subtype) antagonist. Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5- ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one. Its empirical formula is C25H28N6O, and the structural formula: Irbesartan is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water. AVAPRO is available for oral administration in unscored tablets containing 75 mg, 150 mg, or 300 mg of irbesartan. Inactive ingredients include: lactose, microcrystalline cellulose, pregelatinized starch, croscarmellose sodium, poloxamer 188, silicon dioxide and magnesium stearate. CLINICAL PHARMACOLOGY Mechanism of Action Angiotensin II is a potent vasoconstrictor formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system (RAS) and also stimulates aldosterone synthesis and secretion by adrenal NDA 20-757/S-038 Page 4 cortex, cardiac contraction, renal resorption of sodium, activity of the sympathetic nervous system, and smooth muscle cell growth. -

Effects of Olmesartan Vs Irbesartan on Metabolic Parameters and Visfatin in Hypertensive Obese Women
European Review for Medical and Pharmacological Sciences 2010; 14: 759-763 Effects of olmesartan vs irbesartan on metabolic parameters and visfatin in hypertensive obese women D.A. DE LUIS, R. CONDE, M. GONZALEZ SAGRADO, R. ALLER, O. IZAOLA, J.L. PEREZ CASTRILLON, E. ROMERO, M.J. CASTRO Institute of Endocrinology and Nutrition, Medicine School and Unit of Investigation. Hospital Rio Hortega. RD-056/0013 RETICEF. University of Valladolid. Valladolid (Spain) Abstract. – Background: Angiotensin II reg- dence of this rising tide of obesity and associated ulates the production of adipokines. The objective pathologies has led, in the last years, to a dramat- was to study the effect of treatment with irbesartan versus olmesartan in obese hypertensive women. ic increase of researches on the role of adipose Subjects: A sample of 34 obese hypertensive tissue as an active participant in controlling the women was analyzed in a prospective way with a body’s physiology2. randomized trial. Patients were randomized to irbe- Visfatin was recently identified as a protein sartan (300 mg/day) or olmesartan (40 mg/day) for preferentially expressed in visceral adipose tis- 3 months. Weight, body mass index, blood pres- sue, compared with subcutaneous adipose tis- sure, basal glucose, insulin, total cholesterol, LDL- sue3. It can be found in skeletal muscle, liver, cholesterol, HDL-cholesterol, triglycerides, HOMA and visfatin were determined at basal time and af- bone marrow and lymphocytes, where it was ter 3 months of treatment. initially identified as pre-B-cell colony-enhanc- Results: Thirty four patients gave informed con- ing factor (PBEF). Fukuhara et al4 clearly sug- sent and were enrolled in the study. -

AVAPRO Safely and Tablets: 75 Mg, 150 Mg, 300 Mg (3) Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION ———————————— DOSAGE FORMS AND STRENGTHS ———————————— • These highlights do not include all the information needed to use AVAPRO safely and Tablets: 75 mg, 150 mg, 300 mg (3) effectively. See full prescribing information for AVAPRO. AVAPRO® (irbesartan) tablets, for oral use ——————————————— CONTRAINDICATIONS ——————————————— • Hypersensitivity to any component of this product. (4) Initial U.S. Approval: 1997 • Coadministration with aliskiren in patients with diabetes. (4) ————————————— WARNINGS AND PRECAUTIONS ————————————— WARNING: FETAL TOXICITY • Hypotension: Correct volume or salt depletion prior to administration. (5.2) See full prescribing information for complete boxed warning. • Monitor renal function and serum potassium. (5.3) • When pregnancy is detected, discontinue AVAPRO as soon as possible. (5.1, 8.1) • Drugs that act directly on the renin-angiotensin system can cause injury and death ——————————————— ADVERSE REACTIONS ——————————————— to the developing fetus. (5.1, 8.1) • Nephropathy in type 2 diabetic patients: The most common adverse reactions which were more frequent than placebo were hyperkalemia dizziness, orthostatic dizziness, and orthostatic hypotension. (6.1) —————————————— INDICATIONS AND USAGE —————————————— To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800- AVAPRO is an angiotensin II receptor blocker (ARB) indicated for: 633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. • Treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. (1.1) ——————————————— DRUG INTERACTIONS ——————————————— • Treatment of diabetic nephropathy in hypertensive patients with type 2 diabetes, an elevated • Lithium: Risk of lithium toxicity. (7) serum creatinine, and proteinuria. (1.2) • Nonsteroidal Anti-inflammatory Drugs (NSAIDs) and COX-2 inhibitors: Increased risk of renal impairment. -

Irbesartan Hydrochlorothiazide Zentiva, INN-Irbesartan
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Irbesartan Hydrochlorothiazide Zentiva 150 mg/12.5 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 150 mg of irbesartan and 12.5 mg of hydrochlorothiazide. Excipient with known effect: Each tablet contains 26.65 mg of lactose (as lactose monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. Peach, biconvex, oval-shaped, with a heart debossed on one side and the number 2775 engraved on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of essential hypertension. This fixed dose combination is indicated in adult patients whose blood pressure is not adequately controlled on irbesartan or hydrochlorothiazide alone (see section 5.1). 4.2 Posology and method of administration Posology Irbesartan Hydrochlorothiazide Zentiva can be taken once daily, with or without food. Dose titration with the individual components (i.e. irbesartan and hydrochlorothiazide) may be recommended. When clinically appropriate direct change from monotherapy to the fixed combinations may be considered: • Irbesartan Hydrochlorothiazide Zentiva 150 mg/12.5 mg may be administered in patients whose blood pressure is not adequately controlled with hydrochlorothiazide or irbesartan 150 mg alone; • Irbesartan Hydrochlorothiazide Zentiva 300 mg/12.5 mg may be administered in patients insufficiently controlled by irbesartan 300 mg or by Irbesartan Hydrochlorothiazide Zentiva 150 mg/12.5 mg. • Irbesartan Hydrochlorothiazide Zentiva 300 mg/25 mg may be administered in patients insufficiently controlled by Irbesartan Hydrochlorothiazide Zentiva 300 mg/12.5 mg. Doses higher than 300 mg irbesartan/25 mg hydrochlorothiazide once daily are not recommended. -
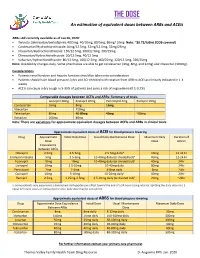
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

COZAAR (Losartan Potassium) Tablets, for Oral Use
HIGHLIGHTS OF PRESCRIBING INFORMATION • Increase dose to 100 mg once daily if further blood pressure These highlights do not include all the information needed to use response is needed. (2.3) COZAAR safely and effectively. See full prescribing information for COZAAR. --------------------- DOSAGE FORMS AND STRENGTHS --------------------- Tablets: 25 mg; 50 mg; and 100 mg. (3) ® COZAAR (losartan potassium) tablets, for oral use ------------------------------- CONTRAINDICATIONS ------------------------------- Initial U.S. Approval: 1995 • Hypersensitivity to any component. (4) • Coadministration with aliskiren in patients with diabetes. (4) WARNING: FETAL TOXICITY See full prescribing information for complete boxed warning. ----------------------- WARNINGS AND PRECAUTIONS ----------------------- • Hypotension: Correct volume or salt depletion prior to administration When pregnancy is detected, discontinue COZAAR as soon as of COZAAR. (5.2) possible. Drugs that act directly on the renin-angiotensin system • Monitor renal function and potassium in susceptible patients. (5.3, can cause injury and death to the developing fetus. (5.1) 5.4) --------------------------- RECENT MAJOR CHANGES --------------------------- ------------------------------ ADVERSE REACTIONS ------------------------------ Warnings and Precautions Hyperkalemia (5.4) 10/2018 Most common adverse reactions (incidence ≥2% and greater than placebo) are: dizziness, upper respiratory infection, nasal congestion, ----------------------------INDICATIONS AND USAGE ---------------------------- and back pain. (6.1) COZAAR is an angiotensin II receptor blocker (ARB) indicated for: • Treatment of hypertension, to lower blood pressure in adults and To report SUSPECTED ADVERSE REACTIONS, contact Merck children greater than 6 years old. Lowering blood pressure reduces Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877- the risk of fatal and nonfatal cardiovascular events, primarily strokes 888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

COZAAR® (LOSARTAN POTASSIUM TABLETS) Page 1 of 28
COZAAR® (LOSARTAN POTASSIUM TABLETS) Page 1 of 28 COZAAR® (LOSARTAN POTASSIUM TABLETS) USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, COZAAR® should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality. DESCRIPTION COZAAR (losartan potassium) is an angiotensin II receptor (type AT1) antagonist. Losartan potassium, a non- peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole- 5-methanol monopotassium salt. Its empirical formula is C22H22ClKN6O, and its structural formula is: Losartan potassium is a white to off-white free-flowing crystalline powder with a molecular weight of 461.01. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone. Oxidation of the 5-hydroxymethyl group on the imidazole ring results in the active metabolite of losartan. COZAAR is available as tablets for oral administration containing either 25 mg, 50 mg or 100 mg of losartan potassium and the following inactive ingredients: microcrystalline cellulose, lactose hydrous, pregelatinized starch, magnesium stearate, hydroxypropyl cellulose, hypromellose, and titanium dioxide. COZAAR 25 mg, 50 mg and 100 mg tablets contain potassium in the following amounts: 2.12 mg (0.054 mEq), 4.24 mg (0.108 mEq) and 8.48 mg (0.216 mEq), respectively. COZAAR 25 mg, COZAAR 50 mg, and COZAAR 100 mg may also contain carnauba wax. -

Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin
DOI: 10.7860/JCDR/2018/31983.11754 Original Article Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin Pharmacology Section Receptor Blockers-A Pilot Study S BHUVANESHWARI1, PRAKASH VEL SANKHYA SAROJ2, D VIJAYA3, M SABARI SOWMYA4, R SENTHIL KUMAR5 ABSTRACT Results: Among all, 48% (24 out of 50) of the study population Introduction: Hyponatremia, serum sodium <135 mmol/L, can administered with ACEI and ARB developed hyponatremia. result in neurological manifestation in acute cases, may lead Predisposition to develop hyponatremia was high in males to seizures and coma. Angiotensin Converting Enzyme Inhibitor compared to females. Incidence of hyponatremia was 62.5% (10 (ACEI) and Angiotensin II Receptor Blockers (ARB) are drugs out of 16) in the age group of 56-75 years. Though, incidence of that have been commonly prescribed for the treatment of hyponatremia was 54.5% (18 out of 33) in ACEI group compared hypertension and cardiac diseases. It has become important to 35.2% (6 out of 17) in ARB group, but it was not statistically to evaluate and investigate the incidence of hyponatremia on significant. The study also revealed that metosartan had a higher consumption of these drugs. association with hyponatremia compared to other drugs. Aim: To determine the susceptibility of patients on ACEI and Conclusion: Hyponatremia was induced in nearly 50% of ARB to hyponatremia and to ascertain the drug producing patients taking ACEI and ARB. The incidence of hyponatremia notable hyponatremia among ACEI and ARB. among patients on these two drugs did not show statistical variation. Metosartan showed a higher incidence of Materials and Methods: The study was conducted in a tertiary hyponatremia compared to enalapril, ramipril, captopril, care hospital. -

Telmisartan Vs Losartan Plus Hydrochlorothiazide in the Treatment of Mild-To-Moderate Essential Hypertensionfa Randomised ABPM Study
Journal of Human Hypertension (2003) 17, 569–575 & 2003 Nature Publishing Group All rights reserved 0950-9240/03 $25.00 www.nature.com/jhh ORIGINAL ARTICLE Telmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertensionFa randomised ABPM study JM Neutel1 , RE Kolloch2, PF Plouin3, TW Meinicke4 and H Schumacher5 for the OTELLOH Study Group 1Orange County Heart Institute & Research Center, Orange, CA, USA; 2Krankenanstalten Gilead, Bielefeld, Germany; 3Hoˆpital Broussais, Paris, France; 4Clinical Research Boehringer Ingelheim Pharma KG, Biberach, Germany; 5Medical Data Services Boehringer Ingelheim Pharma KG, Ingelheim, Germany The objective of this prospective, randomised, open- null hypothesis of a treatment difference of 43 mmHg. label, blinded-end point parallel-group, multicentre The reduction in mean 24-h systolic blood pressure was study was to show that telmisartan 80 mg is not inferior 13.2 7 10.2 mmHg with telmisartan and 17.1 7 10.3 to a fixed-dose combination of losartan 50 mg/hydro- mmHg with losartan/HCTZ. Both drugs provided effec- chlorothiazide (HCTZ) 12.5 mg in patients with mild-to- tive control over the 24-h dosing interval. Analyses of moderate hypertension. The criterion for noninferiority morning (0600–1159) ambulatory blood pressure mon- was a treatment difference of p3.0 mmHg in the reduc- itoring DBP means and trough cuff DBP confirmed the tion of 24-h mean ambulatory diastolic blood pressure noninferiority hypothesis of the protocol for telmisartan (DBP) from the end of the 4-week placebo washout 80 mg vs losartan 50 mg/HCTZ 12.5 mg. The reductions period to the end of the 6-week active treatment period. -

TEVETEN® (Eprosartan Mesylate) 400Mg 600Mg
TEVETEN® (eprosartan mesylate) 400mg 600mg Rx Only PRESCRIBING INFORMATION USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin- angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, TEVETEN® should be discontinued as soon as possible. See WARNINGS: Fetal/Neonatal Morbidity and Mortality. DESCRIPTION ® TEVETEN (eprosartan mesylate) is a non-biphenyl non-tetrazole angiotensin II receptor (AT1) antagonist. A selective non-peptide molecule, TEVETEN® is chemically described as the monomethanesulfonate of (E )-2 butyl-1-(p-carboxybenzyl)-α-2-thienylmethylimid-azole-5-acrylic acid. Its empirical formula is C23H24N2O4S•CH4O3S and molecular weight is 520.625. Its structural formula is: Eprosartan mesylate is a white to off-white free-flowing crystalline powder that is insoluble in water, freely soluble in ethanol, and melts between 248°C and 250°C. TEVETEN® is available as aqueous film-coated tablets containing eprosartan mesylate equivalent to 400 mg or 600 mg eprosartan zwitterion (pink, oval, non-scored tablets or white, non-scored, capsule-shaped tablets, respectively). Dn2262v1-redlined-teveten-2011-mar-14 1 of 14 Reference ID: 2941216 Inactive Ingredients The 400 mg tablet contains the following: croscarmellose sodium, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, and titanium dioxide. The 600 mg tablet contains crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, and titanium dioxide. CLINICAL PHARMACOLOGY Mechanism of Action Angiotensin II (formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme [kininase II]), a potent vasoconstrictor, is the principal pressor agent of the renin-angiotensin system. -

TEVETEN® HCT (Eprosartan Mesylate/Hydrochlorothiazide)600/12.5Mg 600/25Mg Rx Only
TEVETEN HCT - eprosartan mesylate and hydrochlorothiazide tablet Physicians Total Care, Inc. ---------- TEVETEN® HCT (eprosartan mesylate/hydrochlorothiazide)600/12.5mg 600/25mg Rx Only PRESCRIBING INFORMATION USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, TEVETEN® HCT Tablets should be discontinued as soon as possible. See WARNINGS: Fetal/Neonatal Morbidity and Mortality. DESCRIPTION TEVETEN® HCT 600/12.5 mg and TEVETEN® HCT 600/25 mg (eprosartan mesylate- hydrochlorothiazide) combine an angiotensin II receptor (AT1 subtype) antagonist and a diuretic, hydrochlorothiazide. TEVETEN® (eprosartan mesylate) is a non-biphenyl non-tetrazole angiotensin II ® receptor (AT1) antagonist. A selective non-peptide molecule, TEVETEN is chemically described as the monomethanesulfonate of (E)-2-butyl-1-(p-carboxybenzyl)-α-2-thienylmethylimidazole-5-acrylic acid. Its empirical formula is C23H24N2O4S•CH4O3S and molecular weight is 520.625. Its structural formula is: Eprosartan mesylate is a white to off-white free-flowing crystalline powder that is insoluble in water, freely soluble in ethanol, and melts between 248°C and 250°C. Hydrochlorothiazide is 6-chloro-3,4- dihydro-2 H 1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is: Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.74, which is slightly soluble in water, but freely soluble in sodium hydroxide solution. TEVETEN® HCT is available for oral administration in film-coated, non-scored, capsule-shaped tablet combinations of eprosartan mesylate and hydrochlorothiazide.