Effects of Olmesartan Vs Irbesartan on Metabolic Parameters and Visfatin in Hypertensive Obese Women
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AVAPRO Rx Only (Irbesartan) Tablets
NDA 20-757/S-038 Page 3 ® AVAPRO Rx only (irbesartan) Tablets USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, AVAPRO should be discontinued as soon as possible. See WARNINGS: Fetal/Neonatal Morbidity and Mortality. DESCRIPTION ®* AVAPRO (irbesartan) is an angiotensin II receptor (AT1 subtype) antagonist. Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5- ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one. Its empirical formula is C25H28N6O, and the structural formula: Irbesartan is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water. AVAPRO is available for oral administration in unscored tablets containing 75 mg, 150 mg, or 300 mg of irbesartan. Inactive ingredients include: lactose, microcrystalline cellulose, pregelatinized starch, croscarmellose sodium, poloxamer 188, silicon dioxide and magnesium stearate. CLINICAL PHARMACOLOGY Mechanism of Action Angiotensin II is a potent vasoconstrictor formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system (RAS) and also stimulates aldosterone synthesis and secretion by adrenal NDA 20-757/S-038 Page 4 cortex, cardiac contraction, renal resorption of sodium, activity of the sympathetic nervous system, and smooth muscle cell growth. -

BENICAR HCT Tablets
® BENICAR HCT Tablets (OLMESARTAN MEDOXOMIL-HYDROCHLOROTHIAZIDE) WARNING: FETAL TOXICITY When pregnancy is detected, discontinue Benicar HCT as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See Warnings: Fetal Toxicity DESCRIPTION BENICAR HCT® (olmesartan medoxomil-hydrochlorothiazide) is a combination of an angiotensin II receptor antagonist (AT1 subtype), olmesartan medoxomil, and a thiazide diuretic, hydrochlorothiazide (HCTZ). Olmesartan medoxomil, a prodrug, is hydrolyzed to olmesartan during absorption from the gastrointestinal tract. Olmesartan medoxomil is 2,3-dihydroxy-2-butenyl 4-(1-hydroxy-1-methylethyl)-2 propyl-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-carboxylate, cyclic 2,3 carbonate. Its empirical formula is C29H30N6O6 and its structural formula is: Olmesartan medoxomil is a white to light yellowish-white powder or crystalline powder with a molecular weight of 558.6. It is practically insoluble in water and sparingly soluble in methanol. Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzo-thiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is: 1 Reference ID: 3227549 Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.7. Hydrochlorothiazide is slightly soluble in water but freely soluble in sodium hydroxide solution. BENICAR HCT® is available for oral administration in tablets containing 20 mg or 40 mg of olmesartan medoxomil combined with 12.5 mg of hydrochlorothiazide, or 40 mg of olmesartan medoxomil combined with 25 mg of hydrochlorothiazide. Inactive ingredients include: hydroxypropylcellulose, hypromellose, lactose, low-substituted hydroxypropylcellulose, magnesium stearate, microcrystalline cellulose, red iron oxide, talc, titanium dioxide and yellow iron oxide. -

AVAPRO Safely and Tablets: 75 Mg, 150 Mg, 300 Mg (3) Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION ———————————— DOSAGE FORMS AND STRENGTHS ———————————— • These highlights do not include all the information needed to use AVAPRO safely and Tablets: 75 mg, 150 mg, 300 mg (3) effectively. See full prescribing information for AVAPRO. AVAPRO® (irbesartan) tablets, for oral use ——————————————— CONTRAINDICATIONS ——————————————— • Hypersensitivity to any component of this product. (4) Initial U.S. Approval: 1997 • Coadministration with aliskiren in patients with diabetes. (4) ————————————— WARNINGS AND PRECAUTIONS ————————————— WARNING: FETAL TOXICITY • Hypotension: Correct volume or salt depletion prior to administration. (5.2) See full prescribing information for complete boxed warning. • Monitor renal function and serum potassium. (5.3) • When pregnancy is detected, discontinue AVAPRO as soon as possible. (5.1, 8.1) • Drugs that act directly on the renin-angiotensin system can cause injury and death ——————————————— ADVERSE REACTIONS ——————————————— to the developing fetus. (5.1, 8.1) • Nephropathy in type 2 diabetic patients: The most common adverse reactions which were more frequent than placebo were hyperkalemia dizziness, orthostatic dizziness, and orthostatic hypotension. (6.1) —————————————— INDICATIONS AND USAGE —————————————— To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800- AVAPRO is an angiotensin II receptor blocker (ARB) indicated for: 633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. • Treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. (1.1) ——————————————— DRUG INTERACTIONS ——————————————— • Treatment of diabetic nephropathy in hypertensive patients with type 2 diabetes, an elevated • Lithium: Risk of lithium toxicity. (7) serum creatinine, and proteinuria. (1.2) • Nonsteroidal Anti-inflammatory Drugs (NSAIDs) and COX-2 inhibitors: Increased risk of renal impairment. -

Irbesartan Hydrochlorothiazide Zentiva, INN-Irbesartan
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Irbesartan Hydrochlorothiazide Zentiva 150 mg/12.5 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 150 mg of irbesartan and 12.5 mg of hydrochlorothiazide. Excipient with known effect: Each tablet contains 26.65 mg of lactose (as lactose monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. Peach, biconvex, oval-shaped, with a heart debossed on one side and the number 2775 engraved on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of essential hypertension. This fixed dose combination is indicated in adult patients whose blood pressure is not adequately controlled on irbesartan or hydrochlorothiazide alone (see section 5.1). 4.2 Posology and method of administration Posology Irbesartan Hydrochlorothiazide Zentiva can be taken once daily, with or without food. Dose titration with the individual components (i.e. irbesartan and hydrochlorothiazide) may be recommended. When clinically appropriate direct change from monotherapy to the fixed combinations may be considered: • Irbesartan Hydrochlorothiazide Zentiva 150 mg/12.5 mg may be administered in patients whose blood pressure is not adequately controlled with hydrochlorothiazide or irbesartan 150 mg alone; • Irbesartan Hydrochlorothiazide Zentiva 300 mg/12.5 mg may be administered in patients insufficiently controlled by irbesartan 300 mg or by Irbesartan Hydrochlorothiazide Zentiva 150 mg/12.5 mg. • Irbesartan Hydrochlorothiazide Zentiva 300 mg/25 mg may be administered in patients insufficiently controlled by Irbesartan Hydrochlorothiazide Zentiva 300 mg/12.5 mg. Doses higher than 300 mg irbesartan/25 mg hydrochlorothiazide once daily are not recommended. -
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
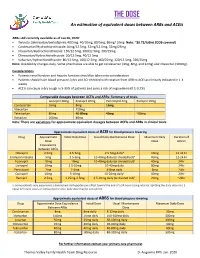
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin
DOI: 10.7860/JCDR/2018/31983.11754 Original Article Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin Pharmacology Section Receptor Blockers-A Pilot Study S BHUVANESHWARI1, PRAKASH VEL SANKHYA SAROJ2, D VIJAYA3, M SABARI SOWMYA4, R SENTHIL KUMAR5 ABSTRACT Results: Among all, 48% (24 out of 50) of the study population Introduction: Hyponatremia, serum sodium <135 mmol/L, can administered with ACEI and ARB developed hyponatremia. result in neurological manifestation in acute cases, may lead Predisposition to develop hyponatremia was high in males to seizures and coma. Angiotensin Converting Enzyme Inhibitor compared to females. Incidence of hyponatremia was 62.5% (10 (ACEI) and Angiotensin II Receptor Blockers (ARB) are drugs out of 16) in the age group of 56-75 years. Though, incidence of that have been commonly prescribed for the treatment of hyponatremia was 54.5% (18 out of 33) in ACEI group compared hypertension and cardiac diseases. It has become important to 35.2% (6 out of 17) in ARB group, but it was not statistically to evaluate and investigate the incidence of hyponatremia on significant. The study also revealed that metosartan had a higher consumption of these drugs. association with hyponatremia compared to other drugs. Aim: To determine the susceptibility of patients on ACEI and Conclusion: Hyponatremia was induced in nearly 50% of ARB to hyponatremia and to ascertain the drug producing patients taking ACEI and ARB. The incidence of hyponatremia notable hyponatremia among ACEI and ARB. among patients on these two drugs did not show statistical variation. Metosartan showed a higher incidence of Materials and Methods: The study was conducted in a tertiary hyponatremia compared to enalapril, ramipril, captopril, care hospital. -

Olmesartan Medoxomil 10 Mg Film-Coated Tablets Olmesartan Medoxomil 20 Mg Film-Coated Tablets Olmesartan Medoxomil 40 Mg Film-Coated Tablets Olmesartan Medoxomil
Package leaflet: Information for the user Olmesartan medoxomil 10 mg Film-coated Tablets Olmesartan medoxomil 20 mg Film-coated Tablets Olmesartan medoxomil 40 mg Film-coated Tablets olmesartan medoxomil Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Olmesartan medoxomil is and what it is used for 2. What you need to know before you take Olmesartan medoxomil 3. How to take Olmesartan medoxomil 4. Possible side effects 5. How to store Olmesartan medoxomil 6. Contents of the pack and other information 1. What Olmesartan medoxomil is and what it is used for Olmesartan medoxomil belongs to a group of medicines called angiotensin-II receptor antagonists. They lower blood pressure by relaxing the blood vessels. This medicine is used for the treatment of high blood pressure (also known as ‘hypertension’) in adults and in children and adolescents aged 6 to less than 18 years. High blood pressure can damage blood vessels in organs such as the heart, kidneys, brain and eyes. In some cases this may lead to a heart attack, heart or kidney failure, stroke or blindness. -

Comparison of Effects of Olmesartan and Telmisartan on Blood Pressure and Metabolic Parameters in Japanese Early-Stage Type-2 Diabetics with Hypertension
7 Hypertens Res Vol.31 (2008) No.1 p.7-13 Original Article Comparison of Effects of Olmesartan and Telmisartan on Blood Pressure and Metabolic Parameters in Japanese Early-Stage Type-2 Diabetics with Hypertension Shiho NAKAYAMA1), Hirotaka WATADA1), Tomoya MITA1), Fuki IKEDA1), Tomoaki SHIMIZU1), Hiroshi UCHINO1), Yoshio FUJITANI1),2), Takahisa HIROSE1),2), and Ryuzo KAWAMORI1),2) Angiotensin II type-1 receptor blockers (ARBs) are regarded as first-line treatments for type-2 diabetes with hypertension. Despite the availability of various types of ARBs, there are no comparative studies of their effects on patients with diabetes. In this open-label prospective crossover study, we compared the effects of olmesartan (20 mg/day) and telmisartan (40 mg/day). Twenty Japanese early-stage type-2 diabetes patients with hypertension treated with valsartan (80 mg/day) for at least 8 weeks were recruited to this study. At study entry, valsartan was changed to olmesartan (20 mg/day) or telmisartan (40 mg/day) and administered for 8 weeks. The drugs were then switched and treatment was continued for another 8 weeks. We analyzed the blood pressure lowering effects of each drug by 24-h ambulatory blood pressure monitor- ing at 0, 8, and 16 weeks. Simultaneously, we measured metabolic parameters and inflammation markers. Olmesartan lowered mean systolic and diastolic blood pressure more significantly than did telmisartan. While there were no differences between the groups in metabolic parameters, including HbA1c and adi- ponectin, the decreases in serum interleukin-6 and highly sensitive C-reactive protein were more significant by olmesartan treatment. Our results indicate that olmesartan has more potent arterial blood pressure low- ering and anti-inflammatory effects than telmisartan. -

Therapeutic Class Overview Angiotensin II Receptor Blockers (Arbs) – Combination Products
Therapeutic Class Overview Angiotensin II receptor blockers (ARBs) – combination products Therapeutic Class • Overview/Summary: This review will focus on the angiotensin II receptor blocker (ARB) combination products.1-13 The renin-angiotensin-aldosterone system (RAAS) is the most important component in the homeostatic regulation of blood pressure.14,15 Excessive activity of the RAAS may lead to hypertension and disorders of fluid and electrolyte imbalance.16 Renin catalyzes the conversion of angiotensinogen to angiotensin I. Angiotensin I is then cleaved to angiotensin II by angiotensin converting enzyme (ACE). Angiotensin II can increase blood pressure by direct vasoconstriction and through actions on the brain and autonomic nervous system.14,16 In addition, angiotensin II stimulates aldosterone synthesis from the adrenal cortex, leading to sodium and water reabsorption. Angiotensin II exerts other detrimental cardiovascular effects including hypertrophy and remodeling.14,15 The RAAS plays an important role in the development and progression of heart failure.15 ACE inhibitors block the conversion of angiotensin I to angiotensin II, and also inhibit the breakdown of bradykinin, a potent vasodilator associated with dry cough.14-17 Since angiotensin II may also be generated through other pathways that do not depend upon ACE (e.g., chymase), blockade of angiotensin II by ACE inhibitors is incomplete.14,15 The ARBs block the angiotensin II receptor subtype AT1, preventing the negative effects of angiotensin II, regardless of its origin. ARBs do not appear to affect bradykinin. Amlodipine, a nondihydropyridine calcium channel blocker, inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Cardiac and vascular smooth muscle contraction depends on the movement of extracellular calcium ions into cells through specific ion channels. -

(Sartans) Article 31 Referral
14 February 2019 EMA/217823/2019 Committee for Medicinal Products for Human Use (CHMP) Assessment report Referral under Article 31 of Directive 2001/83/EC angiotensin-II-receptor antagonists (sartans) containing a tetrazole group Procedure no: EMEA/H/A-31/1471 Nationally authorised products: various Centrally authorised products: Amlodipine-Valsartan Mylan EMEA/H/A-31/1471/C/4037/0004; Aprovel EMEA/H/A- 31/1471/C/141/0172; Coaprovel EMEA/H/A-31/1471/C/222/0187; Copalia EMEA/H/A- 31/1471/C/774/0099; Copalia HCT EMEA/H/A-31/1471/C/1159/0069; Dafiro EMEA/H/A- 31/1471/C/776/0101; Dafiro HCT EMEA/H/A-31/1471/C/1160/0070; Entresto EMEA/H/A- 31/1471/C/4062/0021; Exforge EMEA/H/A-31/1471/C/716/0098; Exforge HCT EMEA/H/A- 31/1471/C/1068/0068; Ifirmacombi EMEA/H/A-31/1471/C/2302/0020; Ifirmasta EMEA/H/A- 31/1471/C/962/0018; Irbesartan Hydrochlorothiazide Zentiva EMEA/H/A-31/1471/C/783/0101; Irbesartan Teva EMEA/H/A-31/1471/C/1093/0032; Irbesartan Zentiva EMEA/H/A- 31/1471/C/785/0080; Irbesartan/Hydrochlorothiazide Teva EMEA/H/A-31/1471/C/1112/0041; Karvea EMEA/H/A-31/1471/C/142/0175; Karvezide EMEA/H/A-31/1471/C/221/0188; Neparvis EMEA/H/A- 31/1471/C/4343/0020 Active substances: candesartan, irbesartan, losartan, olmesartan, valsartan Note: Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2019. -

Product Information for Olmetec (Olmesartan Medoxomil)
Attachment 1: Product information for AusPAR Olmetec Merck Sharp & Dohme (Australia) Pty Ltd PM-2011-01941-3-3 Final 26 February 2013. This Product Information was approved at the time this AusPAR was published. APPROVED PRODUCT INFORMATION OLMETEC (olmesartan medoxomil) NAME OF THE MEDICINE OLMETEC (olmesartan medoxomil), a prodrug, is hydrolyzed to olmesartan during absorption from the gastrointestinal tract. Olmesartan is a selective AT1 subtype angiotensin II receptor antagonist. Olmesartan medoxomil (CAS no. 144689-63-4) is described chemically as 2,3-dihydroxy-2- butenyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl] imidazole-5-carboxylate, cyclic 2,3-carbonate. Its empirical formula is C29H30N6O6 and its structural formula is: DESCRIPTION Olmesartan medoxomil is a white to light yellowish-white powder or crystalline powder with a molecular weight of 558.59. It is practically insoluble in water and sparingly soluble in methanol. OLMETEC is available for oral use as film-coated tablets containing 10 mg, 20 mg, or 40 mg olmesartan medoxomil. OLMETEC tablets also contain the following inactive ingredients: microcrystalline cellulose, low-substituted hydroxypropylcellulose, lactose, hydroxypropylcellulose, magnesium stearate, and Opadry OY-S-38956 that contains titanium dioxide, talc, and hydroxypropylmethylcellulose. OLMETEC extemporaneous suspension contains additional inactive ingredients: purified water, Ora-Sweet® (syrup vehicle) and Ora-Plus® (suspending vehicle). Ora-Sweet® contains citric acid, flavouring, glycerine, methylparaben, potassium sorbate, sodium phosphate, sorbitol, sucrose, and purified water. Ora-Plus® contains calcium sulphate, carrageenan, citric acid, dimethicone antifoam emulsion, methylparaben, microcrystalline cellulose, sodium carboxymethylcellulose, potassium sorbate, sodium phosphate monobasic, trisodium phosphate, xanthan gum, and purified water. -
Irbesartan 150 Mg Tablets
Your doctor may check your kidney function, blood Package leaflet: pressure, and the amount of electrolytes (e.g. Information for the user potassium) in your blood at regular intervals. Irbesartan 75 mg tablets See also information under the heading “Do not take Irbesartan 150 mg tablets Irbesartan” Irbesartan 300 mg tablets You must tell your doctor if you think you are (or might become) pregnant. Irbesartan is not Irbesartan recommended in early pregnancy, and must not be taken if you are more than 3 months pregnant, as it may cause serious harm to your baby if used at that stage (see pregnancy section). Read all of this leaflet carefully before you Children and adolescents start taking this medicine because it contains This medicinal product should not be used in children important information for you. and adolescents because the safety and efficacy - Keep this leaflet. You may need to read it again. have not yet been fully established. - If you have any further questions, ask your doctor or pharmacist. Other medicines and Irbesartan - This medicine has been prescribed for you only. Tell your doctor or pharmacist if you are taking, have Do not pass it on to others. It may harm them, recently taken or might take any other medicines. even if their signs of illness are the same as Your doctor may need to change your dose and/or to yours. take other precautions: - If you get any side effects, talk to your doctor or pharmacist. This includes any possible side If you are taking an ACE-inhibitor or aliskiren (see effects not listed in this leaflet.