(Sartans) Article 31 Referral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AVAPRO Rx Only (Irbesartan) Tablets
NDA 20-757/S-038 Page 3 ® AVAPRO Rx only (irbesartan) Tablets USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, AVAPRO should be discontinued as soon as possible. See WARNINGS: Fetal/Neonatal Morbidity and Mortality. DESCRIPTION ®* AVAPRO (irbesartan) is an angiotensin II receptor (AT1 subtype) antagonist. Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5- ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one. Its empirical formula is C25H28N6O, and the structural formula: Irbesartan is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water. AVAPRO is available for oral administration in unscored tablets containing 75 mg, 150 mg, or 300 mg of irbesartan. Inactive ingredients include: lactose, microcrystalline cellulose, pregelatinized starch, croscarmellose sodium, poloxamer 188, silicon dioxide and magnesium stearate. CLINICAL PHARMACOLOGY Mechanism of Action Angiotensin II is a potent vasoconstrictor formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system (RAS) and also stimulates aldosterone synthesis and secretion by adrenal NDA 20-757/S-038 Page 4 cortex, cardiac contraction, renal resorption of sodium, activity of the sympathetic nervous system, and smooth muscle cell growth. -

Effects of Olmesartan Vs Irbesartan on Metabolic Parameters and Visfatin in Hypertensive Obese Women
European Review for Medical and Pharmacological Sciences 2010; 14: 759-763 Effects of olmesartan vs irbesartan on metabolic parameters and visfatin in hypertensive obese women D.A. DE LUIS, R. CONDE, M. GONZALEZ SAGRADO, R. ALLER, O. IZAOLA, J.L. PEREZ CASTRILLON, E. ROMERO, M.J. CASTRO Institute of Endocrinology and Nutrition, Medicine School and Unit of Investigation. Hospital Rio Hortega. RD-056/0013 RETICEF. University of Valladolid. Valladolid (Spain) Abstract. – Background: Angiotensin II reg- dence of this rising tide of obesity and associated ulates the production of adipokines. The objective pathologies has led, in the last years, to a dramat- was to study the effect of treatment with irbesartan versus olmesartan in obese hypertensive women. ic increase of researches on the role of adipose Subjects: A sample of 34 obese hypertensive tissue as an active participant in controlling the women was analyzed in a prospective way with a body’s physiology2. randomized trial. Patients were randomized to irbe- Visfatin was recently identified as a protein sartan (300 mg/day) or olmesartan (40 mg/day) for preferentially expressed in visceral adipose tis- 3 months. Weight, body mass index, blood pres- sue, compared with subcutaneous adipose tis- sure, basal glucose, insulin, total cholesterol, LDL- sue3. It can be found in skeletal muscle, liver, cholesterol, HDL-cholesterol, triglycerides, HOMA and visfatin were determined at basal time and af- bone marrow and lymphocytes, where it was ter 3 months of treatment. initially identified as pre-B-cell colony-enhanc- Results: Thirty four patients gave informed con- ing factor (PBEF). Fukuhara et al4 clearly sug- sent and were enrolled in the study. -

AVAPRO Safely and Tablets: 75 Mg, 150 Mg, 300 Mg (3) Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION ———————————— DOSAGE FORMS AND STRENGTHS ———————————— • These highlights do not include all the information needed to use AVAPRO safely and Tablets: 75 mg, 150 mg, 300 mg (3) effectively. See full prescribing information for AVAPRO. AVAPRO® (irbesartan) tablets, for oral use ——————————————— CONTRAINDICATIONS ——————————————— • Hypersensitivity to any component of this product. (4) Initial U.S. Approval: 1997 • Coadministration with aliskiren in patients with diabetes. (4) ————————————— WARNINGS AND PRECAUTIONS ————————————— WARNING: FETAL TOXICITY • Hypotension: Correct volume or salt depletion prior to administration. (5.2) See full prescribing information for complete boxed warning. • Monitor renal function and serum potassium. (5.3) • When pregnancy is detected, discontinue AVAPRO as soon as possible. (5.1, 8.1) • Drugs that act directly on the renin-angiotensin system can cause injury and death ——————————————— ADVERSE REACTIONS ——————————————— to the developing fetus. (5.1, 8.1) • Nephropathy in type 2 diabetic patients: The most common adverse reactions which were more frequent than placebo were hyperkalemia dizziness, orthostatic dizziness, and orthostatic hypotension. (6.1) —————————————— INDICATIONS AND USAGE —————————————— To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800- AVAPRO is an angiotensin II receptor blocker (ARB) indicated for: 633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. • Treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. (1.1) ——————————————— DRUG INTERACTIONS ——————————————— • Treatment of diabetic nephropathy in hypertensive patients with type 2 diabetes, an elevated • Lithium: Risk of lithium toxicity. (7) serum creatinine, and proteinuria. (1.2) • Nonsteroidal Anti-inflammatory Drugs (NSAIDs) and COX-2 inhibitors: Increased risk of renal impairment. -

Irbesartan Hydrochlorothiazide Zentiva, INN-Irbesartan
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Irbesartan Hydrochlorothiazide Zentiva 150 mg/12.5 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 150 mg of irbesartan and 12.5 mg of hydrochlorothiazide. Excipient with known effect: Each tablet contains 26.65 mg of lactose (as lactose monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. Peach, biconvex, oval-shaped, with a heart debossed on one side and the number 2775 engraved on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of essential hypertension. This fixed dose combination is indicated in adult patients whose blood pressure is not adequately controlled on irbesartan or hydrochlorothiazide alone (see section 5.1). 4.2 Posology and method of administration Posology Irbesartan Hydrochlorothiazide Zentiva can be taken once daily, with or without food. Dose titration with the individual components (i.e. irbesartan and hydrochlorothiazide) may be recommended. When clinically appropriate direct change from monotherapy to the fixed combinations may be considered: • Irbesartan Hydrochlorothiazide Zentiva 150 mg/12.5 mg may be administered in patients whose blood pressure is not adequately controlled with hydrochlorothiazide or irbesartan 150 mg alone; • Irbesartan Hydrochlorothiazide Zentiva 300 mg/12.5 mg may be administered in patients insufficiently controlled by irbesartan 300 mg or by Irbesartan Hydrochlorothiazide Zentiva 150 mg/12.5 mg. • Irbesartan Hydrochlorothiazide Zentiva 300 mg/25 mg may be administered in patients insufficiently controlled by Irbesartan Hydrochlorothiazide Zentiva 300 mg/12.5 mg. Doses higher than 300 mg irbesartan/25 mg hydrochlorothiazide once daily are not recommended. -
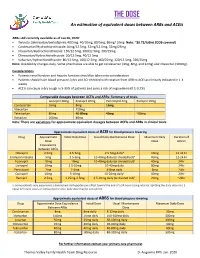
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin
DOI: 10.7860/JCDR/2018/31983.11754 Original Article Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin Pharmacology Section Receptor Blockers-A Pilot Study S BHUVANESHWARI1, PRAKASH VEL SANKHYA SAROJ2, D VIJAYA3, M SABARI SOWMYA4, R SENTHIL KUMAR5 ABSTRACT Results: Among all, 48% (24 out of 50) of the study population Introduction: Hyponatremia, serum sodium <135 mmol/L, can administered with ACEI and ARB developed hyponatremia. result in neurological manifestation in acute cases, may lead Predisposition to develop hyponatremia was high in males to seizures and coma. Angiotensin Converting Enzyme Inhibitor compared to females. Incidence of hyponatremia was 62.5% (10 (ACEI) and Angiotensin II Receptor Blockers (ARB) are drugs out of 16) in the age group of 56-75 years. Though, incidence of that have been commonly prescribed for the treatment of hyponatremia was 54.5% (18 out of 33) in ACEI group compared hypertension and cardiac diseases. It has become important to 35.2% (6 out of 17) in ARB group, but it was not statistically to evaluate and investigate the incidence of hyponatremia on significant. The study also revealed that metosartan had a higher consumption of these drugs. association with hyponatremia compared to other drugs. Aim: To determine the susceptibility of patients on ACEI and Conclusion: Hyponatremia was induced in nearly 50% of ARB to hyponatremia and to ascertain the drug producing patients taking ACEI and ARB. The incidence of hyponatremia notable hyponatremia among ACEI and ARB. among patients on these two drugs did not show statistical variation. Metosartan showed a higher incidence of Materials and Methods: The study was conducted in a tertiary hyponatremia compared to enalapril, ramipril, captopril, care hospital. -

Therapeutic Class Overview Angiotensin II Receptor Blockers (Arbs) – Combination Products
Therapeutic Class Overview Angiotensin II receptor blockers (ARBs) – combination products Therapeutic Class • Overview/Summary: This review will focus on the angiotensin II receptor blocker (ARB) combination products.1-13 The renin-angiotensin-aldosterone system (RAAS) is the most important component in the homeostatic regulation of blood pressure.14,15 Excessive activity of the RAAS may lead to hypertension and disorders of fluid and electrolyte imbalance.16 Renin catalyzes the conversion of angiotensinogen to angiotensin I. Angiotensin I is then cleaved to angiotensin II by angiotensin converting enzyme (ACE). Angiotensin II can increase blood pressure by direct vasoconstriction and through actions on the brain and autonomic nervous system.14,16 In addition, angiotensin II stimulates aldosterone synthesis from the adrenal cortex, leading to sodium and water reabsorption. Angiotensin II exerts other detrimental cardiovascular effects including hypertrophy and remodeling.14,15 The RAAS plays an important role in the development and progression of heart failure.15 ACE inhibitors block the conversion of angiotensin I to angiotensin II, and also inhibit the breakdown of bradykinin, a potent vasodilator associated with dry cough.14-17 Since angiotensin II may also be generated through other pathways that do not depend upon ACE (e.g., chymase), blockade of angiotensin II by ACE inhibitors is incomplete.14,15 The ARBs block the angiotensin II receptor subtype AT1, preventing the negative effects of angiotensin II, regardless of its origin. ARBs do not appear to affect bradykinin. Amlodipine, a nondihydropyridine calcium channel blocker, inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Cardiac and vascular smooth muscle contraction depends on the movement of extracellular calcium ions into cells through specific ion channels. -
Irbesartan 150 Mg Tablets
Your doctor may check your kidney function, blood Package leaflet: pressure, and the amount of electrolytes (e.g. Information for the user potassium) in your blood at regular intervals. Irbesartan 75 mg tablets See also information under the heading “Do not take Irbesartan 150 mg tablets Irbesartan” Irbesartan 300 mg tablets You must tell your doctor if you think you are (or might become) pregnant. Irbesartan is not Irbesartan recommended in early pregnancy, and must not be taken if you are more than 3 months pregnant, as it may cause serious harm to your baby if used at that stage (see pregnancy section). Read all of this leaflet carefully before you Children and adolescents start taking this medicine because it contains This medicinal product should not be used in children important information for you. and adolescents because the safety and efficacy - Keep this leaflet. You may need to read it again. have not yet been fully established. - If you have any further questions, ask your doctor or pharmacist. Other medicines and Irbesartan - This medicine has been prescribed for you only. Tell your doctor or pharmacist if you are taking, have Do not pass it on to others. It may harm them, recently taken or might take any other medicines. even if their signs of illness are the same as Your doctor may need to change your dose and/or to yours. take other precautions: - If you get any side effects, talk to your doctor or pharmacist. This includes any possible side If you are taking an ACE-inhibitor or aliskiren (see effects not listed in this leaflet. -

Comparison of the Acute Antihypertensive Response to Telmisartan and Irbesartan in Spontaneously Hypertensive Rats
ORIGINAL ARTICLE CME Comparison of the Acute Antihypertensive Response to Telmisartan and Irbesartan in Spontaneously Hypertensive Rats Comparación de la respuesta antihipertensiva aguda al telmisartán y al irbesartán en ratas espontáneamente hipertensas MATÍAS LUCERO1, YANINA SANTANDER1, LUCIANO PAROLA1, JULIETA S. DEL MAURO1, MARCELA MORETÓN2, FACUNDO M. BERTERA1,3, DIEGO CHIAPPETTA2, CHRISTIAN HÖCHT1,3, CARLOS A. TAIRA1,3 ABSTRACT Background: Telmisartan and irbesartan, two of the main AT1 receptor antagonists available for the control of cardiovascular diseases, differ in their pharmacological properties, including time of dissociation from the AT1 receptor and the ability to activate other receptors, with potential impact on their relative clinical efficacy. Objectives: The aim of this study was to compare the acute cardiovascular response to single dose administration of irbesartan or telmisartan in spontaneously hypertensive rats. Methods: Twenty-four male spontaneously hypertensive rats, weighing 250-275 g, were used. The carotid artery and femoral vein were cannulated for direct mean arterial pressure measurement (MAP) and irbesartan 3-6 mg/kg or telmisartan 0,5-1 mg/kg admin- istration. Changes in MAP, heart rate and short-term and beat-to-beat blood pressure variability were estimated. Results: Although both antagonists reduced MAP, telmisartan induced a longer antihypertensive response than irbesartan, evi- denced by greater MAP reduction after 180 min (-33.3%±4.1% vs. -16.3%±4%; p<0.05). Telmisartan and irbesartan induced sus- tained reduction of short-term blood pressure variability without significant differences between both experimental groups. At the lower dose level, telmisartan produced greater decrease of heart rate and beat-to-beat blood pressure variability at the different frequency domains compared with irbesartan. -

Review of Existing Classification Efforts
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.1.1 Review of existing classification efforts Due date of deliverable: (15.01.2008) Actual submission date: (07.02.2008) Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UGent Revision 1.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public X PP Restricted to other programme participants (including the Commission Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) Task 4.1 : Review of existing classification efforts Authors: Kristof Pil, Elke Raes, Thomas Van den Neste, An-Sofie Goessaert, Jolien Veramme, Alain Verstraete (Ghent University, Belgium) Partners: - F. Javier Alvarez (work package leader), M. Trinidad Gómez-Talegón, Inmaculada Fierro (University of Valladolid, Spain) - Monica Colas, Juan Carlos Gonzalez-Luque (DGT, Spain) - Han de Gier, Sylvia Hummel, Sholeh Mobaser (University of Groningen, the Netherlands) - Martina Albrecht, Michael Heiβing (Bundesanstalt für Straßenwesen, Germany) - Michel Mallaret, Charles Mercier-Guyon (University of Grenoble, Centre Regional de Pharmacovigilance, France) - Vassilis Papakostopoulos, Villy Portouli, Andriani Mousadakou (Centre for Research and Technology Hellas, Greece) DRUID 6th Framework Programme Deliverable D.4.1.1. Revision 1.0 Review of Existing Classification Efforts Page 2 of 127 Introduction DRUID work package 4 focusses on the classification and labeling of medicinal drugs according to their influence on driving performance. -

Comparative Efficacy and Safety of Aliskiren and Irbesartan in Patients with Hypertension and Metabolic Syndrome
Journal of Human Hypertension (2011) 25, 186–195 & 2011 Macmillan Publishers Limited All rights reserved 0950-9240/11 www.nature.com/jhh ORIGINAL ARTICLE Comparative efficacy and safety of aliskiren and irbesartan in patients with hypertension and metabolic syndrome W Krone1, M Hanefeld2, H-F Meyer3, T Jung4, M Bartlett5, C-M Yeh6, I Rajman5, MF Prescott6 and WP Dole7 1Klinik II und Poliklinik fu¨r Innere Medizin, Zentrum fu¨r Molekulare Medizin der Universita¨tzuKo¨ln, Cologne, Germany; 2Centre for Clinical Studies, GWT-TUD GmbH, Dresden, Germany; 3Facharzt fu¨r Allgemeinmedizin, Marl, Germany; 4Facharzt fu¨r Allgemeinmedizin, Deggingen, Germany; 5Novartis Institutes for Biomedical Research, Basel, Switzerland; 6Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA and 7Novartis Institutes for Biomedical Research Inc., Cambridge, MA, USA Metabolic syndrome, a cluster of risk factors that than the 5.8/2.8 mm Hg reduction observed in patients increase the risk of cardiovascular morbidity and mor- treated with irbesartan 300 mg. A significantly greater tality, is common in patients with hypertension. Chronic proportion of patients treated with aliskiren achieved BP renin–angiotensin–aldosterone system (RAAS) activa- control to o135/85 mm Hg (29.2 vs 16.7% with irbesartan; tion, shown by elevated plasma renin activity (PRA), is P ¼ 0.019). Aliskiren treatment led to a 60% decrease in implicated in many of the features of metabolic syn- PRA from baseline, whereas irbesartan increased PRA drome. The direct renin inhibitor aliskiren may be of by 99% (both Po0.001). Aliskiren and irbesartan had benefit in this patient group as aliskiren targets the similar effects on glucose and lipid profiles and on a RAAS at the rate-limiting step. -

Free PDF Download
European Review for Medical and Pharmacological Sciences 2013; 17: 410-419 Pattern analysis and variations in the utilization of antihypertensive drugs in Taiwan: a six-year study L.-Y. HUANG1,2, W.-Y. SHAU1, H.-C. CHEN3, S. SU2, M.-C. YANG2, H.-L. YEH4,5, M.-S. LAI3,6 1Division of Health Technology Assessment, Center for Drug Evaluation, Taipei, Taiwan 2Graduate Institute of Health Care Organization Administration, College of Public Health, National Taiwan University, Taipei, Taiwan 3Graduate Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan 4Department of Cardiology, Sijhih Cathay General Hospital, Taipei, Taiwan 5School of Public Health, College of Public Health and Nutrition, Taipei Medical University, Taipei, Taiwan 6Center for Comparative Effectiveness Research, National Clinical Trial and Research Center, National Taiwan University Hospital, Taipei, Taiwan Abstract. – BACKGROUND: In the last few CONCLUSIONS: The consumption of antihy- years there have been changed in the pattern of pertension drugs in Taiwan increased during the consumption of antihypertensive drugs in other period studied and the highest average annual countries. Factors causing this variability in- increases were for ARBs and CCBs. Overall con- clude differences in the effectiveness of detec- sumption of antihypertension drugs also in- tion, guidelines for the management of hyperten- creased in other countries, but differences in the sion, and differences in national health insur- relative increase for each class of drug suggest ance systems among countries. that further study may be required to clarify the AIM: The aim of this study was to reveal pat- origins and causes. terns in the use of antihypertensive drugs in Tai- wan over a six year period (2001 to 2006) and com- Key Words: pare these results with data from other countries.