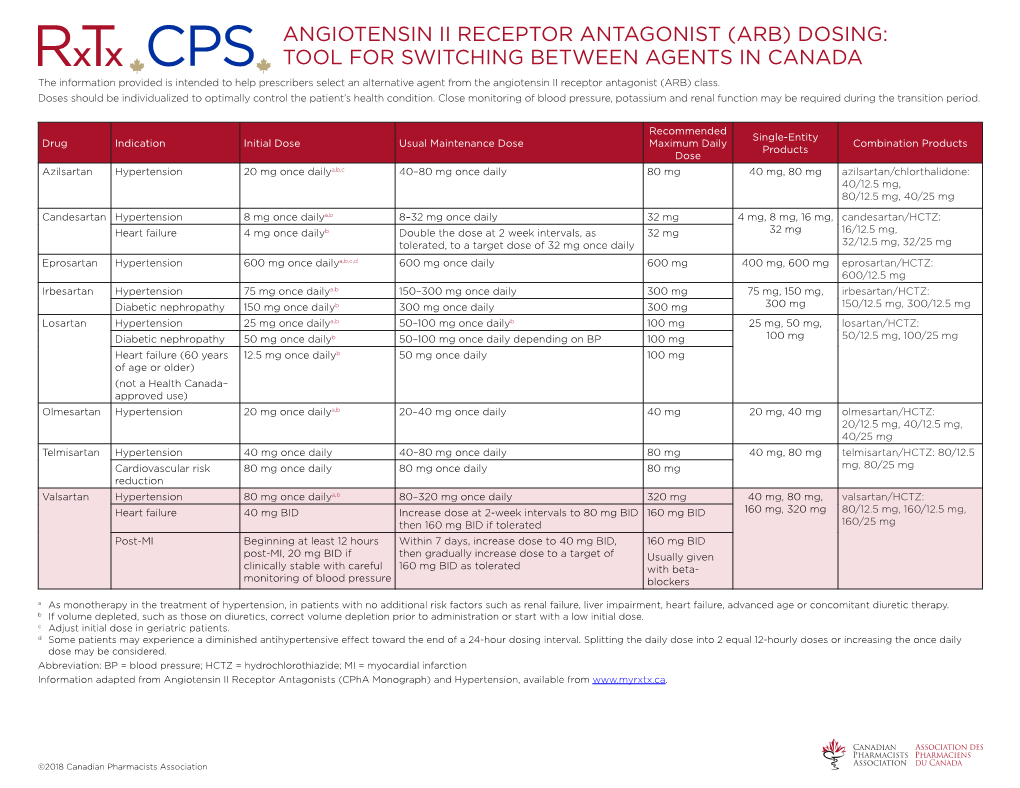
Angiotensin Ii Receptor Antagonist (Arb) Dosing: Tool for Switching Between Agents in Canada
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Key Features of Candesartan Cilexetil and a Comparison with Other Angiotensin II Receptor Antagonists
Journal of Human Hypertension (1999) 13, (Suppl 1), S3–S10 1999 Stockton Press. All rights reserved 0950-9240/99 $12.00 Key features of candesartan cilexetil and a comparison with other angiotensin II receptor antagonists PS Sever Imperial College of Science, Technology & Medicine at St Mary’s Hospital, London, UK Current research on angiotensin II AT1-receptor antag- in patients with essential hypertension. Candesartan onists (AIIRAs) and selected studies presented at the cilexetil has a rapid onset of action (approximately 80% recent symposium held in Amsterdam, The Netherlands, of total blood pressure reduction within the first 2 on 6 June 1998, titled ‘Angiotensin II Receptor Antagon- weeks) and dose-dependent effects on blood pressure, ists are NOT all the Same’ are reviewed. AIIRAs offer a is comparable in efficacy to a number of classes of anti- number of potential advantages over alternative antihy- hypertensives, and is effective in combination therapy pertensive agents acting via the renin-angiotensin-aldo- (eg, with hydrochlorothiazide and amlodipine). This sterone system. They combine blood pressure-lowering favourable profile may be due in part to the highly selec- effects at least equivalent to those of angiotensin-con- tive, tight binding to and slow dissociation of candesar- verting enzyme (ACE) inhibitors, coupled with placebo- tan from the AT1 receptor. Preliminary studies suggest like tolerability. Candesartan cilexetil is a novel AIIRA that candesartan cilexetil also protects end organs that has demonstrated clinical -

Diovan® Valsartan
NDA 21-283/S-011 Page 3 T2005-02 Diovan® valsartan Tablets Rx only Prescribing Information USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, Diovan should be discontinued as soon as possible. See WARNINGS: Fetal/Neonatal Morbidity and Mortality. DESCRIPTION Diovan® (valsartan) is a nonpeptide, orally active, and specific angiotensin II antagonist acting on the AT1 receptor subtype. Valsartan is chemically described as N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl) [1,1′-biphenyl]- 4-yl]methyl]-L-valine. Its empirical formula is C24H29N5O3, its molecular weight is 435.5, and its structural formula is Valsartan is a white to practically white fine powder. It is soluble in ethanol and methanol and slightly soluble in water. NDA 21-283/S-011 Page 4 Diovan is available as tablets for oral administration, containing 40 mg, 80 mg, 160 mg or 320 mg of valsartan. The inactive ingredients of the tablets are colloidal silicon dioxide, crospovidone, hydroxypropyl methylcellulose, iron oxides (yellow, black and/or red), magnesium stearate, microcrystalline cellulose, polyethylene glycol 8000, and titanium dioxide. CLINICAL PHARMACOLOGY Mechanism of Action Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Valsartan blocks the vasoconstrictor and aldosterone- secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. -

Unique Binding Behavior of the Recently Approved Angiotensin II Receptor Blocker Azilsartan Compared with That of Candesartan
Hypertension Research (2013) 36, 134–139 & 2013 The Japanese Society of Hypertension All rights reserved 0916-9636/13 www.nature.com/hr ORIGINAL ARTICLE Unique binding behavior of the recently approved angiotensin II receptor blocker azilsartan compared with that of candesartan Shin-ichiro Miura1,2,3, Atsutoshi Okabe4, Yoshino Matsuo1, Sadashiva S Karnik3 and Keijiro Saku1,2 The angiotensin II type 1 (AT1) receptor blocker (ARB) candesartan strongly reduces blood pressure (BP) in patients with hypertension and has been shown to have cardioprotective effects. A new ARB, azilsartan, was recently approved and has been shown to provide a more potent 24-h sustained antihypertensive effect than candesartan. However, the molecular interactions of azilsartan with the AT1 receptor that could explain its strong BP-lowering activity are not yet clear. To address this issue, we examined the binding affinities of ARBs for the AT1 receptor and their inverse agonist activity toward the production of inositol phosphate (IP), and we constructed docking models for the interactions between ARBs and the receptor. Azilsartan, unlike candesartan, has a unique moiety, a 5-oxo-1,2,4-oxadiazole, in place of a tetrazole ring. Although the results regarding the binding affinities of azilsartan and candesartan demonstrated that these ARBs interact with the same sites in the AT1 receptor (Tyr113,Lys199 and Gln257), the hydrogen bonding between the oxadiazole of azilsartan-Gln257 is stronger than that between the tetrazole of candesartan-Gln257, according to molecular docking models. An examination of the inhibition of IP production by ARBs using constitutively active mutant receptors indicated that inverse agonist activity required azilsartan– Gln257 interaction and that azilsartan had a stronger interaction with Gln257 than candesartan. -

AVAPRO Rx Only (Irbesartan) Tablets
NDA 20-757/S-038 Page 3 ® AVAPRO Rx only (irbesartan) Tablets USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, AVAPRO should be discontinued as soon as possible. See WARNINGS: Fetal/Neonatal Morbidity and Mortality. DESCRIPTION ®* AVAPRO (irbesartan) is an angiotensin II receptor (AT1 subtype) antagonist. Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5- ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one. Its empirical formula is C25H28N6O, and the structural formula: Irbesartan is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water. AVAPRO is available for oral administration in unscored tablets containing 75 mg, 150 mg, or 300 mg of irbesartan. Inactive ingredients include: lactose, microcrystalline cellulose, pregelatinized starch, croscarmellose sodium, poloxamer 188, silicon dioxide and magnesium stearate. CLINICAL PHARMACOLOGY Mechanism of Action Angiotensin II is a potent vasoconstrictor formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system (RAS) and also stimulates aldosterone synthesis and secretion by adrenal NDA 20-757/S-038 Page 4 cortex, cardiac contraction, renal resorption of sodium, activity of the sympathetic nervous system, and smooth muscle cell growth. -

Annexes to the EMA Annual Report 2009
Annual report 2009 Annexes The main body of this annual report is available on the website of the European Medicines Agency (EMA) at: http://www.ema.europa.eu/htms/general/direct/ar.htm 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E-mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2010. Reproduction is authorised provided the source is acknowledged. Contents Annex 1 Members of the Management Board..................................................... 3 Annex 2 Members of the Committee for Medicinal Products for Human Use .......... 5 Annex 3 Members of the Committee for Medicinal Products for Veterinary Use ....... 8 Annex 4 Members of the Committee on Orphan Medicinal Products .................... 10 Annex 5 Members of the Committee on Herbal Medicinal Products ..................... 12 Annex 6 Members of the Paediatric Committee................................................ 14 Annex 7 National competent authority partners ............................................... 16 Annex 8 Budget summaries 2008–2009 ......................................................... 27 Annex 9 European Medicines Agency Establishment Plan .................................. 28 Annex 10 CHMP opinions in 2009 on medicinal products for human use .............. 29 Annex 11 CVMP opinions in 2009 on medicinal products for veterinary use.......... 53 Annex 12 COMP opinions in 2009 on designation of orphan medicinal products -
Download Leaflet View the Patient Leaflet in PDF Format
Package leaflet: Information for the user Candesartan cilexetil 2 mg tablets Candesartan cilexetil 4 mg tablets Candesartan cilexetil 8 mg tablets Candesartan cilexetil 16 mg tablets Candesartan cilexetil 32 mg tablets candesartan cilexetil Read all of this leaflet carefully If you are going to have an operation, before you start taking this tell your doctor or dentist that you are medicine because it contains taking Candesartan cilexetil. This is important information for you. because Candesartan cilexetil, when - Keep this leaflet. You may need combined with some anaesthetics, to read it again. may cause an excessive drop in - If you have any further questions, blood pressure. ask your doctor or pharmacist. - This medicine has been Children and adolescents prescribed for you only. Do not Candesartan Cilexetil has been pass it on to others. It may harm studied in children. For more them, even if their signs of illness information, talk to your doctor. are the same as yours. Candesartan Cilexetil must not be - If you get any side effects, talk to given to children under 1 year of your doctor or pharmacist. This age due to the potential risk to the includes any possible side effects developing kidneys. not listed in this leaflet. See Other medicines and Candesartan section 4. cilexetil What is in this leaflet Tell your doctor or pharmacist if you 1. What Candesartan cilexetil is and are taking, have recently taken or what it is used for might take any other medicines. 2. What you need to know before you take Candesartan cilexetil Candesartan cilexetil can affect the 3. -

BENICAR HCT Tablets
® BENICAR HCT Tablets (OLMESARTAN MEDOXOMIL-HYDROCHLOROTHIAZIDE) WARNING: FETAL TOXICITY When pregnancy is detected, discontinue Benicar HCT as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See Warnings: Fetal Toxicity DESCRIPTION BENICAR HCT® (olmesartan medoxomil-hydrochlorothiazide) is a combination of an angiotensin II receptor antagonist (AT1 subtype), olmesartan medoxomil, and a thiazide diuretic, hydrochlorothiazide (HCTZ). Olmesartan medoxomil, a prodrug, is hydrolyzed to olmesartan during absorption from the gastrointestinal tract. Olmesartan medoxomil is 2,3-dihydroxy-2-butenyl 4-(1-hydroxy-1-methylethyl)-2 propyl-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-carboxylate, cyclic 2,3 carbonate. Its empirical formula is C29H30N6O6 and its structural formula is: Olmesartan medoxomil is a white to light yellowish-white powder or crystalline powder with a molecular weight of 558.6. It is practically insoluble in water and sparingly soluble in methanol. Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzo-thiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is: 1 Reference ID: 3227549 Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.7. Hydrochlorothiazide is slightly soluble in water but freely soluble in sodium hydroxide solution. BENICAR HCT® is available for oral administration in tablets containing 20 mg or 40 mg of olmesartan medoxomil combined with 12.5 mg of hydrochlorothiazide, or 40 mg of olmesartan medoxomil combined with 25 mg of hydrochlorothiazide. Inactive ingredients include: hydroxypropylcellulose, hypromellose, lactose, low-substituted hydroxypropylcellulose, magnesium stearate, microcrystalline cellulose, red iron oxide, talc, titanium dioxide and yellow iron oxide. -

Edarbi Generic Name: Azilsartan Manufacturer1
Brand Name: Edarbi Generic Name: azilsartan Manufacturer1: Takeda Pharmaceutical Company Drug Class1,2,3,4,5: Anti‐hypertensive, Angiotensin II receptor blocker Uses: Labeled Uses1,2,3,4,5: Hypertension, alone or as adjunctive therapy with other antihypertensives Unlabeled Uses: 1,2,3,4,5 Mechanism of Action : Azilsartan inhibits the binding of angiotensin II to AT1 receptor. This blocks the vasoconstrictor and aldosterone‐secreting effects of angiotensin II. Pharmacokinetics1,2,3,4,5: Absorption: Tmax 1.5‐3 hours Vd 16 L t1/2 11 hours Clearance 2.3 mL/min Protein binding >99% Bioavailability 60% Metabolism: Azilsartan is metabolized via O‐dealkylation and decarboxylation into two primary metabolites. CYP2C9 is the major enzyme responsible for azilsartan metabolism. Elimination: Azilsartan is eliminated through the feces (55%) and the urine (42%). Only 15% is eliminated as the unchanged drug. Efficacy: Bakris GL, Sica D, Weber M, White WB, Roberts A, Perez A, Cao C, et al. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. Journal of Clinical Hypertension. 2001;13(2):81‐88. Study Design: Randomized, double‐blind, multicenter, parallel group, placebo‐controlled study design Description of Study: Methods: One thousand two hundred seventy‐five patients meeting the criteria of primary hypertension were randomized to receive either placebo, 40mg of olmesartan, or 20mg, 40mg, or 80mg of azilsartan for six weeks. The primary end point, change in 24 hour mean systolic blood pressure at week six, was assessed by ambulatory blood pressure monitoring performed at baseline and the final visit. The key secondary endpoint was change in trough sitting clinic systolic blood pressure at week six. -

Efficacy and Safety of Azilsartan Medoxomil, an Angiotensin Receptor
Juhasz et al. Clinical Hypertension (2018) 24:2 DOI 10.1186/s40885-018-0086-4 RESEARCH Open Access Efficacy and safety of azilsartan medoxomil, an angiotensin receptor blocker, in Korean patients with essential hypertension Attila Juhasz1,5, Jingtao Wu2, Michie Hisada2, Tomoka Tsukada3,6 and Myung Ho Jeong4* Abstract Background: This was a phase 3, randomized, double-blind, placebo-controlled study. Methods: Adult Korean patients with essential hypertension and a baseline mean sitting clinic systolic blood pressure (scSBP) ≥150 and ≤180 mmHg were randomized to 6-week treatment with placebo (n = 65), azilsartan medoxomil (AZL-M) 40 mg (n = 132), or AZL-M 80 mg (n = 131). The primary endpoint was the change from baseline to week 6 in trough scSBP. Results: The least-squares mean (standard error) change from baseline in trough scSBP in the placebo, AZL-M 40-mg, and 80-mg groups at week 6 were − 8.8 (2.00), − 22.1 (1.41), and − 23.7 (1.40) mmHg, respectively (p < 0.001 for AZL-M 40 and 80 mg vs placebo). No clinically meaningful heterogeneity in efficacy was observed between subgroups (age, sex, diabetes status) and the overall population. Treatments were well tolerated and adverse events were similar between groups. Conclusions: Results of this study confirm a positive benefit-risk profile of AZL-M for essential hypertension in Korean adults. Trial registration: Clinicaltrial.gov; identifier number: NCT02203916. Registered July 28, 2014 (retrospectively registered) Keywords: Hypertension, Azilsartan medoxomil, Angiotensin II receptor antagonist, Blood pressure, Korea Background cerebrovascular and cardiovascular disease, which are now Hypertension is a leading cause of preventable death in the second and third most common causes of death in developed nations and of increasing prevalence in develop- Korea, respectively [11]. -

Effects of Olmesartan Vs Irbesartan on Metabolic Parameters and Visfatin in Hypertensive Obese Women
European Review for Medical and Pharmacological Sciences 2010; 14: 759-763 Effects of olmesartan vs irbesartan on metabolic parameters and visfatin in hypertensive obese women D.A. DE LUIS, R. CONDE, M. GONZALEZ SAGRADO, R. ALLER, O. IZAOLA, J.L. PEREZ CASTRILLON, E. ROMERO, M.J. CASTRO Institute of Endocrinology and Nutrition, Medicine School and Unit of Investigation. Hospital Rio Hortega. RD-056/0013 RETICEF. University of Valladolid. Valladolid (Spain) Abstract. – Background: Angiotensin II reg- dence of this rising tide of obesity and associated ulates the production of adipokines. The objective pathologies has led, in the last years, to a dramat- was to study the effect of treatment with irbesartan versus olmesartan in obese hypertensive women. ic increase of researches on the role of adipose Subjects: A sample of 34 obese hypertensive tissue as an active participant in controlling the women was analyzed in a prospective way with a body’s physiology2. randomized trial. Patients were randomized to irbe- Visfatin was recently identified as a protein sartan (300 mg/day) or olmesartan (40 mg/day) for preferentially expressed in visceral adipose tis- 3 months. Weight, body mass index, blood pres- sue, compared with subcutaneous adipose tis- sure, basal glucose, insulin, total cholesterol, LDL- sue3. It can be found in skeletal muscle, liver, cholesterol, HDL-cholesterol, triglycerides, HOMA and visfatin were determined at basal time and af- bone marrow and lymphocytes, where it was ter 3 months of treatment. initially identified as pre-B-cell colony-enhanc- Results: Thirty four patients gave informed con- ing factor (PBEF). Fukuhara et al4 clearly sug- sent and were enrolled in the study. -

AVAPRO Safely and Tablets: 75 Mg, 150 Mg, 300 Mg (3) Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION ———————————— DOSAGE FORMS AND STRENGTHS ———————————— • These highlights do not include all the information needed to use AVAPRO safely and Tablets: 75 mg, 150 mg, 300 mg (3) effectively. See full prescribing information for AVAPRO. AVAPRO® (irbesartan) tablets, for oral use ——————————————— CONTRAINDICATIONS ——————————————— • Hypersensitivity to any component of this product. (4) Initial U.S. Approval: 1997 • Coadministration with aliskiren in patients with diabetes. (4) ————————————— WARNINGS AND PRECAUTIONS ————————————— WARNING: FETAL TOXICITY • Hypotension: Correct volume or salt depletion prior to administration. (5.2) See full prescribing information for complete boxed warning. • Monitor renal function and serum potassium. (5.3) • When pregnancy is detected, discontinue AVAPRO as soon as possible. (5.1, 8.1) • Drugs that act directly on the renin-angiotensin system can cause injury and death ——————————————— ADVERSE REACTIONS ——————————————— to the developing fetus. (5.1, 8.1) • Nephropathy in type 2 diabetic patients: The most common adverse reactions which were more frequent than placebo were hyperkalemia dizziness, orthostatic dizziness, and orthostatic hypotension. (6.1) —————————————— INDICATIONS AND USAGE —————————————— To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800- AVAPRO is an angiotensin II receptor blocker (ARB) indicated for: 633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. • Treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. (1.1) ——————————————— DRUG INTERACTIONS ——————————————— • Treatment of diabetic nephropathy in hypertensive patients with type 2 diabetes, an elevated • Lithium: Risk of lithium toxicity. (7) serum creatinine, and proteinuria. (1.2) • Nonsteroidal Anti-inflammatory Drugs (NSAIDs) and COX-2 inhibitors: Increased risk of renal impairment. -

Blocking the Tissue Renin-Angiotensin System: the Future Cornerstone of Therapy
Journal of Human Hypertension (2000) 14, Suppl 2, S23–S31 2000 Macmillan Publishers Ltd All rights reserved 0950-9240/00 $15.00 www.nature.com/jhh Blocking the tissue renin-angiotensin system: the future cornerstone of therapy T Unger1, M Azizi2 and GG Belz3 1Institute of Pharmacology, Christian Albrechts University, Hospitalstrae 4, 24105 Kiel, Germany; 2Centre D’Investigations Cliniques, Hoˆ pital Broussais, 96 Rue Didot – 75674, Paris Cedex 14, France; 3Zentrum fuer Kardiovaskulaere Pharmakologie, ZeKaPha GmbH, Mathildenstrae 8, D-55116, Mainz-Wiesbaden, Germany The development of angiotensin-converting enzyme antagonist, is characterised by its tight binding to and inhibitors and selective angiotensin type 1 (AT1)- slow dissociation from the AT1 receptor, and high antag- receptor antagonists has provided new insights into onistic potency, resulting in long-lasting antagonistic understanding the mechanism of the renin-angiotensin effects. It is anticipated that these pharmacological system (RAS) in the pathophysiology of cardiovascular characteristics may bring additional benefits to patients, disease. There is good evidence from meta-analyses not only for the management of essential hypertension that shows that inhibition of the RAS achieves organ but also for the management of end-organ damage. protection features that go beyond blood pressure con- Journal of Human Hypertension (2000) 14, Suppl 2, S23– trol. Candesartan cilexetil, a new angiotensin II receptor S31 Keywords: renin-angiotensin system; angiotensin-converting enzyme inhibitor; angiotensin type 1 receptor; angiotensin receptor antagonist; candesartan cilexetil Introduction Blocking the RAS in essential Hypertension is a major risk factor for myocardial hypertension infarction, stroke, and renal and peripheral vascular Angiotensin II, the key effector peptide of the RAS, disease.