Common Glossary of Terms Used in Medicines Registration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Immunology New Orleans, LA Toll Free 888.355.4191 Toll Free Fax 888.355.4192 Krogerspecialtypharmacy.Com
Immunology New Orleans, LA toll free 888.355.4191 toll free fax 888.355.4192 krogerspecialtypharmacy.com Need By Date: _________________________________________________ Ship To: Patient Office Fax Copy: Rx Card Front/Back Clinical Notes Medical Card Front/Back Patient Information Prescriber Information Patient Name Prescriber Name Address Address City State Zip City State Zip Main Phone Alternate Phone Phone Fax Social Security # Contact Person Date of Birth Male Female DEA # NPI # License # Clinical Information Diagnosis: J45.40 Moderate Asthma J45.50 Severe Asthma L20.9 Atopic Dermatitis L50.1 Chronic Idiopathic Urticaria (CIU) Eosinophil Levels J33 Chronic Rhinosinusitis with Nasal Polyposis Other: ________________________________ Dx Code: ___________ Drug Allergies Concomitant Therapies: Short-acting Beta Agonist Long-acting Beta Agonist Antihistamines Decongestants Immunotherapy Inhaled Corticosteroid Leukotriene Modifiers Oral Steroids Nasal Steroids Other: _____________________________________________________________ Please List Therapies Weight kg lbs Date Weight Obtained Lab Results: History of positive skin OR RAST test to a perennial aeroallergen Pretreatment Serum lgE Level: ______________________________________ IU per mL Test Date: _________ / ________ / ________ MD Specialty: Allergist Dermatologist ENT Pediatrician Primary Care Prescription Type: Naïve/New Start Restart Continued Therapy Pulmonologist Other: _________________________________________ Last Injection Date: _________ / ________ -

STEM Disciplines
STEM Disciplines In order to be applicable to the many types of institutions that participate in the HERI Faculty Survey, this list is intentionally broad and comprehensive in its definition of STEM disciplines. It includes disciplines in the life sciences, physical sciences, engineering, mathematics, computer science, and the health sciences. Agriculture/Natural Resources Health Professions 0101 Agriculture and related sciences 1501 Alternative/complementary medicine/sys 0102 Natural resources and conservation 1503 Clinical/medical lab science/allied 0103 Agriculture/natural resources/related, other 1504 Dental support services/allied 1505 Dentistry Biological and Biomedical Sciences 1506 Health & medical administrative services 0501 Biochem/biophysics/molecular biology 1507 Allied health and medical assisting services 0502 Botany/plant biology 1508 Allied health diagnostic, intervention, 0503 Genetics treatment professions 0504 Microbiological sciences & immunology 1509 Medicine, including psychiatry 0505 Physiology, pathology & related sciences 1511 Nursing 0506 Zoology/animal biology 1512 Optometry 0507 Biological & biomedical sciences, other 1513 Osteopathic medicine/osteopathy 1514 Pharmacy/pharmaceutical sciences/admin Computer/Info Sciences/Support Tech 1515 Podiatric medicine/podiatry 0801 Computer/info tech administration/mgmt 1516 Public health 0802 Computer programming 1518 Veterinary medicine 0803 Computer science 1519 Health/related clinical services, other 0804 Computer software and media applications 0805 Computer systems -

Pharmacy/Medical Drug Prior Authorization Form
High Cost Medical Drugs List High Cost Medical Drugs administered by Health Alliance™ providers within physician offices, infusion centers or hospital outpatient settings must be acquired from preferred specialty vendors. Health Alliance will not reimburse any drug listed as a “High Cost Medical Drug,” whether obtained from the provider’s own stock or via “buy-and-bill.” This drug list does not apply to members with Medicare coverage. Information on how to acquire these medications is located at the end of this document. Recent Updates Preferred Contact Drug Therapy Drug Name Code PA Effective Change Vendor Number Oncology – Injectable DARZALEX FASPRO J9144 YES 10/1/2021 CVS/Caremark® 800-237-2767 Added High Cost Medical Drug List Preferred Contact Drug Therapy Drug Name Code PA Effective Vendor Number Acromegaly SANDOSTATIN J2353 YES 7/1/2020 CVS/Caremark® 800-237-2767 Acromegaly SOMATULINE J1930 YES 7/1/2020 CVS/Caremark® 800-237-2767 Additional Products JETREA J7316 YES 7/1/2020 LDD Additional Products PROLASTIN J0256 YES 7/1/2020 LDD Additional Products QUTENZA J7336 NO 7/1/2020 LDD Additional Products REVCOVI J3590 YES 7/1/2020 LDD Additional Products RADICAVA J1301 YES 7/1/2020 CVS/Caremark® 800-237-2767 Additional Products SIGNIFOR J2502 YES 7/1/2020 Accredo® 866-759-1557 Additional Products SPRAVATO J3490 YES 7/1/2020 CVS/Caremark® 800-237-2767 Additional Products STRENSIQ J3590 YES 7/1/2020 LDD Additional Products THIOTEPA J9340 YES 7/1/2020 CVS/Caremark® 800-237-2767 Allergic Asthma CINQAIR J2786 YES 7/1/2020 CVS/Caremark® 800-237-2767 -

Current Status of Immunology Education in US Schools And
American Journal of Pharmaceutical Education 2019; 83 (7) Article 6994. RESEARCH Current Status of Immunology Education in US Schools and Colleges of Pharmacy Yuan Zhao, PhD,a Dana Ho, PharmD,a Benjamin Oldham, PharmD,a Bonnie Dong, PharmD,a Daniel Malcom, PharmDa,b a Sullivan University College of Pharmacy, Louisville, Kentucky b Associate Editor, American Journal of Pharmaceutical Education, Arlington, Virginia Submitted February 2, 2018; accepted June 17, 2018; published September 2019. Objective. To determine the extent of immunology education in US Doctor of Pharmacy (PharmD) programs. Methods. Curricular information on immunology education was collected from the web pages of US PharmD programs (N5142). The data were sorted, comparisons were made, and trends were identified. Results. Of 142 PharmD programs studied, 100% posted curriculum information on their websites. Among them, 73 programs (51.4%) had a dedicated immunology course in their curriculum, either as an independent course or a course combined with another subject. Most immunology education was offered in the first professional year (72.5%). Of the programs that offered immunology as an in- dependent course, the number of semester hours dedicated to the course varied from 1.5 to 3.5 (median53, mode53, mean52.7). More three-year programs offered immunology as a core compo- nent in the didactic curriculum than did four-year programs (64.7% vs 49.6%). Similarly, more private programs offered immunology than did public programs (64% vs 37.3%). Conclusion. Immunology education in US schools and colleges of pharmacy lacks consensus. Not all PharmD programs indicated they offered specific, focused immunology education in their curricula. -

Telepharmacy Rules and Statutes: a 50-State Survey
American Journal of Medical Research 5(2), 2018 pp. 7–23, ISSN 2334-4814, eISSN 2376-4481 doi:10.22381/AJMR5220181 TELEPHARMACY RULES AND STATUTES: A 50-STATE SURVEY GEORGE TZANETAKOS Department of Health Management and Policy, College of Public Health, The University of Iowa FRED ULLRICH Department of Health Management and Policy, College of Public Health, The University of Iowa KEITH MUELLER [email protected] Department of Health Management and Policy, College of Public Health, The University of Iowa (corresponding author) ABSTRACT. There has been a significant decline in the number of independently owned rural pharmacies serving non-metropolitan areas, thereby limiting access to pharmaceutical services for rural residents, particularly for those most vulnerable and in need of these services. The use of telepharmacy is one potential solution to this problem. Telepharmacies deliver pharmaceutical care to outpatients at a distance via telecommunication and other advanced technologies. This study identifies rules and laws enacted by states authorizing the use of community telepharmacy initiatives within their respective jurisdictions. As of August 2016, the use of telepharmacy was authorized, in varying capacities, in 23 states (46%). Pilot program development that could apply to telepharmacy initiatives was authorized by six states (12%). Waivers to administrative or legislative pharmacy practice requirements that could allow for telepharmacy initiatives were permitted in five states (10%). Nearly one-third of the states (16, or 32%) did not authorize the use of telepharmacy, nor did they authorize the pursuit of telepharmacy initiatives via pilot programs or waivers. Keywords: telepharmacy; rule; statute; rural; community; outpatient How to cite: Tzanetakos, George, Fred Ullrich, and Keith Mueller (2018). -

Health Science: Biomedicine Public Service Endorsement
INNOVATE • COLLABORATE • EDUCATE • INNOVATE • COLLABORATE • EDUCATE Health Science: Biomedicine Public Service Endorsement Career Pathways • Biochemist • Biomedical Engineer • Biophysicist • Physician, Surgeon Certifications / Certificate Options • CPR • OSHA Program Highlights Biomedical engineering represents new areas of medical research and • Pathogen Analysis product development; biomedical engineers’ work helps pave the way • Human Anatomy & Physiology for new ways to help treat injuries and diseases. As medicine is a field • DNA / Genomics with vast numbers of specific disciplines, there are many different sub- • Laboratory Procedures fields in which biomedical engineers work. Some work to improve and • Dissection develop new machinery, such as robotic surgery equipment; others • Grow and Culture Bacteria endeavor to create better, more reliable replacement limbs (or parts • Real World Research which help existing limbs function better, such as joint replacements). • Learn to Treat Patients New and more comfortable patient beds, monitoring equipment, and electronics are also products that often begin as concepts from the CTSO(s) biomedical engineer’s or involve some level of input from them. • HOSA - Future Health Professionals KISD Biomedical Program: Program Fees / Requirements Biomedicine classes in KISD aim to prepare students to enter the • HOSA membership (Optional) health care field by giving them a foundation in real world problems, medical treatments, and a better understanding of the human body. Program Location Students will investigate causes and treatments of diseases that R Course(s) available at CHS affect the entire world as well as ones found in our own community. R Course(s) available at FRHS Students learn advanced techniques used in hospitals and research R Course(s) available at KHS labs and will become familiar with protocols used by doctors and R Course(s) available at TCHS researchers today. -

Specialty Pharmacy Drug List
Specialty Pharmacy Drug List Our Specialty Pharmacy provides patients with comprehensive support services and coordinated delivery related to high-cost oral, inhaled or injectable specialty medications, used to treat complex conditions. We are your single source for high-touch patient care management to control side effects, patient support and education to ensure compliance or continued treatment, and specialized handling and distribution of medications directly to the patient or care provider. Specialty medications may be covered under either the medical or pharmacy benefit. Please consult your insurance documentation to determine which benefit covers these medications. We offer a broad specialty medication list containing nearly 500 drugs, covering 42 therapeutic categories and specialty disease states. This list is updated with new information each quarter. Characteristics of Specialty Medications “Specialty” medications are defined as high-cost oral or injectable medications used to treat complex chronic conditions. These are highly complex medications, typically biology-based, that structurally mimic compounds found within the body. High-touch patient care management is usually required to control side effects and ensure compliance. Specialized handling and distribution are also necessary to ensure appropriate medication administration. Medications must have at least one of the following characteristics in order to be classified as a specialty medication by Magellan Rx Management. High Cost High Complexity High Touch High-cost medications -

Biomedicine, Pharmacy and Health Care SECTORAL SERVICES of MATHEMATICAL RESEARCH Spanish Network for Mathematics & Industry for COMPANIES C/ Lope Gómez De Marzoa, S/N
Biomedicine, pharmacy and health care SECTORAL SERVICES OF MATHEMATICAL RESEARCH Spanish Network for Mathematics & Industry FOR COMPANIES C/ Lope Gómez de Marzoa, s/n. Campus Vida | CP 15782 Santiago de Compostela (A Coruña) Tel. 881 813 373 | 881 813 223 [email protected] | www.math-in.net math- in Biomedicine, Pharmacy and Health Care Specialized services Mathematics is an effective tool with proven ability to Biomedicine, Pharmacy and Health industries bring meet the demands and needs of businesses. In Spain, together the supply of 29 research groups of the i-MATH Biomedicine and Pharmacy many innovative solutions emerged from the experience project, of which up to 20 have proven experience from gained by research groups in this field through successful having effectively carried out consulting activities, training • Analysis and design of experiments. • Studies of efficacy and safety of treatments. partnerships with industry over the years. All those courses and other collaborations. The details of these • Characterization/grading of pharmaceutical and medical waste. encouraging cases support its potential in offering an groups can be found in the 'Sectors' section of the • Modelling of mortality tables. increasingly sophisticated and complete service to the platform www.math-in.net. • Numerical simulation of fractures, dental implants and orthodontic brackets. industry. • Biomechanics. Bones formation. The mathematical technology services provided in this • Bioinformatics. • Mathematical Biology. Synthetic Biology. The Spanish Network for Mathematics & Industry sector can be broken down into two blocks (on the one • Computational design of proteins. (math-in) is a stable platform that continues the work hand, Biomedicine and Pharmacy and, on the other • Metabolism. -

Planning for Pharmacy School
CENTER FOR CAREER & PROFESSIONAL DEVELOPMENT Planning for Pharmacy School Why Pharmacy? nutrition support. Pharmacists have also been instrumental in establishing many of the poison information and control centers Pharmacy is a career that offers great benefits, flexible work across the country. schedules, outstanding growth opportunities, profit sharing, and much more. If you enjoy working with people, excel in science, and would like a rewarding healthcare career, you may want to Career Options in Pharmacy: consider pharmacy. Academic pharmacy A well-rounded career: Pharmacy is a blend of science, Community practice healthcare, direct patient contact, computer technology, and business. Government agencies A vital part of the healthcare system: Pharmacists play an Hospice and home care integral role in improving patient’s health through the Hospital and institutional practice medicine and information they provide. Long-term care or consulting pharmacy Outstanding opportunities: There is an increasing need for Managed care pharmacy pharmacists in a wide variety of occupational settings. Medical and scientific publishing Excellent earning potential: Pharmacy is one of the most Pharmaceutical industry/sciences financially rewarding careers. Pharmacy law A trusted profession: Pharmacists are consistently ranked as Trade or professional associations one of the most highly trusted professionals because of the care and service they provide (according to data by Wirthlin Uniformed (public health) services Worldwide and Gallup International). Many pharmacists spend most of their workday on their feet. Community and hospital pharmacies are often open for extended The Pharmacy Profession hours or around the clock, so pharmacists may work nights, Although pharmacists are known as professionals whose primary weekends, and holidays. -
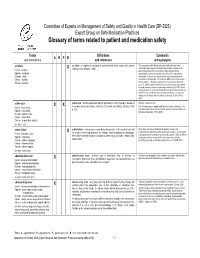
Glossary of Terms Related to Patient and Medication Safety
Committee of Experts on Management of Safety and Quality in Health Care (SP-SQS) Expert Group on Safe Medication Practices Glossary of terms related to patient and medication safety Terms Definitions Comments A R P B and translations and references and synonyms accident accident : an unplanned, unexpected, and undesired event, usually with adverse “For many years safety officials and public health authorities have Xconsequences (Senders, 1994). discouraged use of the word "accident" when it refers to injuries or the French : accident events that produce them. An accident is often understood to be Spanish : accidente unpredictable -a chance occurrence or an "act of God"- and therefore German : Unfall unavoidable. However, most injuries and their precipitating events are Italiano : incidente predictable and preventable. That is why the BMJ has decided to ban the Slovene : nesreča word accident. (…) Purging a common term from our lexicon will not be easy. "Accident" remains entrenched in lay and medical discourse and will no doubt continue to appear in manuscripts submitted to the BMJ. We are asking our editors to be vigilant in detecting and rejecting inappropriate use of the "A" word, and we trust that our readers will keep us on our toes by alerting us to instances when "accidents" slip through.” (Davis & Pless, 2001) active error X X active error : an error associated with the performance of the ‘front-line’ operator of Synonym : sharp-end error French : erreur active a complex system and whose effects are felt almost immediately. (Reason, 1990, This definition has been slightly modified by the Institute of Medicine : “an p.173) error that occurs at the level of the frontline operator and whose effects are Spanish : error activo felt almost immediately.” (Kohn, 2000) German : aktiver Fehler Italiano : errore attivo Slovene : neposredna napaka see also : error active failure active failures : actions or processes during the provision of direct patient care that Since failure is a term not defined in the glossary, its use is not X recommended. -

Allied Health Professionals! Learn More
Have a healthcare career Did you know. without being a nurse or doctor! that you could have a healthcare career without being a nurse or a doctor? In fact, most people in healthcare belong to another Learn more. group of professionals called The Health Professions Network is made allied health providers. These up of different allied health professional professionals work to deliver groups. You can learn more about allied health and specific careers in allied health high-quality patient care servic- by visiting the Health Professions Network es for the identification, preven- website on the Internet. You can also tion and treatment of diseases, download more copies of this brochure and print an allied health poster. disabilities and disorders. That’s a long way of saying that allied The address is: health providers work to make www.healthpronet.org sick or injured people healthy You can also explore allied health careers and keep them healthy. by visiting schools that have allied health educational programs. To find out more There are HUNDREDS of allied about the schools you can visit these web- sites: It’s challenging… health professions and over six million people in allied health For the National Network of Health Career jobs! That means that most peo- Programs in Two-Year Colleges ple in healthcare are not doctors www.NN2.org or nurses, but they are. For the Association of Schools of Allied 0906-1343 Health Professions www.asahp.org Allied Health Professionals! It’s cutting edge… It’s rewarding… Allied Common Questions ? Health How long do I have to go to school? How much will I get paid? You MUST finish high school! After that, it How much you get paid depends on which job Careers depends on the job you do how long you’ll need to you choose to do and how long you go to? school. -

Medical School Personal Statement
Sample Before Editing Medical School Personal Statement My friend’s father always warned him not to run a lot since he had asthma. While playing games he always stopped to use an inhaler, which eventually enabled him to run for a while. As a child I was fascinated by how small particles in that inhaler could treat his big lungs. He recovered from that terrifying infirmity, but imprinted in my mind was the feeling of relief he felt from the inhaled drug, which his life depended on. What began as a childhood fascination became an ambition for the rest of my life. As I was enrolled at the University of Houston in 2009 pursuing my undergraduate degree in Health Science, I wanted to further my knowledge beyond the classroom setting. For this reason, I decided to work as a pharmacy technician to obtain a sense of what my professional life might be like. I observed the day-to-day performance of the pharmacists, their interaction with the community, and how well they were reciprocated. The experience and knowledge I gained motivated me to continue working as a pharmacy tech even after I graduated. My times spent working, as a pharmacy technician was as educational as it was emotionally worthwhile. The experience gained during my four years working at CVS, Walgreens and at the University of Pittsburgh Medical Center Hospital, allowed me to understand that pharmacists are able to make a difference on their community simply by counseling patients who are experiencing drug- drug, drug-disease, and drug-food interactions. It became apparent that pharmacists served as the last line of defense in protecting patients against any drug-related problems.