Ecosystem Overview and Assessment Report for the Bras D'or Lakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

January 2017 News for Descendants of Johann Christopher Windemuth B
January 2017 News for descendants of Johann Christopher Windemuth b. 1676 Windemuth Family Newsletter Related Family Names: Windemuth*Wintamote*Wintamute*Wintemute*Wintermote*Wintermute*Wintermuth Nancy Lane Washington D.C. Debutante 4th Great Granddaughter of Georg Philip Windemuth Nancy Lane grew up in Washington D.C. when President Theodore Roosevelt and Woodrow Wilson were in office. Her father Franklin Knight Lane, was a commissioner and then Chairmen of the Interstate Commerce Commission. He was then appointed as the 26th Secretary of the Interior by President Wilson. Nancy was born on January 4, 1903 to Anna Clair Wintermute and Franklin Knight Lane, in Los Angeles, California. Her older bother Franklin Knight Lane Jr. was born April 5, 1896 in San Francisco, California. Nancy’s father, Franklin Knight Lane, was born in Charlottetown, Prince Edward Is- land in 1864 and her mother Anna Clair Wintermute born in Ontario, Canada in 1870. Her parent’s were married in Tacoma Washington in 1893 where Franklin Lane was Nancy Lane editor and part owner of the Tacoma Newspaper. Franklin and Anna early life was in Washington D.C. 1918 San Francisco where Franklin was practicing law with is bother. He became San Fran- cisco’s District Attorney and also ran for Governor of California 1902, but lost. Continued on Page 3 Inside this issue: Coming Soon Welcome to Cape Breton 2 Nancy Lane 3 Windemuth Family Reunion Nancy Lane 4 Reunion Registration 5 ****July 10-13, 2017**** Reunion Itinerary 6 This is a great opportunity to renew friendships Heritage Books and 7 With cousins and meet new ones Officers Missing Members 8 Registration forms Life Members 9 are on page 5 and 6 Membership Payments © 2016 Windemuth Family Organization Windemuth Family Newsletter Page 2 January 2017 Welcome to Cape Breton, Nova Scotia by Norma (Wintermute) Marchant Once you arrive in Cape Breton, you will see the phrase, “Ciad Mille Failte!” on signage throughout the island. -

Celtic-Colours-Guide-2019-1
11-19 October 2019 • Cape Breton Island Festival Guide e l ù t h a s a n ò l l g r a t e i i d i r h . a g L s i i s k l e i t a h h e t ò o e c b e , a n n i a t h h a m t o s d u o r e r s o u ’ a n d n s n a o u r r a t I l . s u y l c a g n r a d e h , n t c e , u l n l u t i f u e r h l e t i u h E o e y r r e h a t i i s w d h e e e d v i p l , a a v d i b n r a a t n h c a e t r i a u c ’ a a h t a n a u h c ’ a s i r h c a t l o C WELCOME Message from the Atlantic Canada Message de l’Agence de promotion A Message from the Honourable Opportunities Agency économique du Canada atlantique Stephen McNeil, M.L.A. Premier Welcome to the 2019 Celtic Colours Bienvenue au Celtic Colours On behalf of the Province of Nova International Festival International Festival 2019 Scotia, I am delighted to welcome you to the 2019 Celtic Colours International Tourism is a vital part of the Atlantic Le tourisme est une composante Festival. -

1-888-355-7744 Toll Free 902-567-3000 Local
celtic-colours•com REMOVE MAP TO USE Official Festival Map MAP LEGEND Community Event Icons Meat Cove BAY ST. LAWRENCE | Capstick Official Learning Outdoor Participatory Concert Opportunities Event Event ST. MARGARET'S VILLAGE | ASPY BAY | North Harbour Farmers’ Visual Art / Community Local Food White Point Market Heritage Craft Meal Product CAPE NORTH | Smelt Brook Map Symbols Red River SOUTH HARBOUR | Pleasant Bay Participating Road BIG INTERVALE | Community Lone Shieling NEIL’S HARBOUR | Dirt Road Highway Cabot Trail CAPE BRETON HIGHLANDS NATIONAL PARK Cap Rouge TICKETS & INFORMATION 1-888-355-7744 TOLL FREE Keltic Lodge 902-567-3000 LOCAL CHÉTICAMP | Ingonish Beach INGONISH | Ingonish Ferry La Pointe GRAND ÉTANG HARBOUR | Wreck Cove Terre Noire Skir Dhu BELLE CÔTE | ATLANTIC.CAA.CA French River Margaree Harbour North Shore INDIAN BROOK | Chimney Corner East Margaree MARGAREE CENTER | Tarbotvale NORTH EAST MARGAREE | ENGLISHTOWN | Dunvegan MARGAREE FORKS | Big Bras d’Dor NORTH RIVER | SYDNEY MINES | Lake O’Law 16 BROAD COVE | SOUTH WEST MARGAREE | 17 18 15 Bras d’Dor 19 Victoria NEW WATERFORD | 12 14 20 21 Mines Scotchtown SOUTH HAVEN | 13 Dominion INVERNESS | 2 South Bar GLACE BAY | SCOTSVILLE | MIDDLE RIVER | 11 NORTH SYDNEY | ST. ANN'S | Donkin STRATHLORNE | Big Hill BOULARDERIE | 3 PORT MORIEN | 125 SYDNEY | L 10 Westmount A BADDECK | 4 K Ross Ferry E Barachois A COXHEATH | I MEMBERTOU | N 5 S East Lake Ainslie 8 L I 9 7 E 6 SYDNEY RIVER | WAGMATCOOK7 | HOWIE CENTRE | WEST MABOU | 8 Homeville West Lake Ainslie PRIME BROOK | BOISDALE -

Travelling in Time to Cape Breton Island in the 1920S: Protest Songs, Murals and Island Identity
Travelling in Time to Cape Breton Island in the 1920s: Protest Songs, Murals and Island Identity Richard MacKinnon and Lachlan MacKinnon Abstract Islands are places that foster a unique sense of place-attachment and com- munity identity among their populations. Scholarship focusing on the dis- tinctive values, attitudes and perspectives of ‘island people’ from around the world reveals the layers of meaning that are attached to island life. Lowenthal writes: ‘Islands are fantasized as antitheses of the all-engrossing gargantuan mainstream-small, quiet, untroubled, remote from the busy, crowded, turbu- lent everyday scene. In reality, most of them are nothing like that. …’1 Islands, for many people, are ‘imagined places’ in our increasingly globalised world; the perceptions of island culture and reality often differ. Cape Breton Island, Nova Scotia, in eastern North America, a locale with a rich history of class struggle surrounding its former coal and steel industries, provides an excellent case study for the ways that local history, collective memory and cultural expression might combine to combat the ‘untroubled fantasy’ that Lowenthal describes. History and methodology Coal mining has been an essential part of Cape Breton Island’s landscape since the early-eighteenth century. A steel mill was constructed in Sydney, the island’s largest city, in 1899; this steel plant provided employment for many of the island’s inhabitants throughout the twentieth century. Grid-patterned streets, dotted with company-owned homes, formed around the industrial workplaces in many Cape Breton communities. It was in these communities, from the people employed in the coal mines and steel mill, that distinctive traditions of work and leisure began to emerge. -

Sangamonian Forest History and Climate in Atlantic Canada
Document generated on 09/24/2021 8:37 a.m. Géographie physique et Quaternaire Sangamonian Forest History and Climate in Atlantic Canada L’évolution de la forêt et du climat au Sangamonien dans l'est du Canada Entwicklung des Waldes und des Klimas im Sangamonium, atlantisches Kanada Robert J. Mott The Last? Interglaciation in Canada Article abstract Le dernier (?) interglaciaire au Canada Seven of the more than twenty five buried organic deposits in Atlantic Canada Volume 44, Number 3, 1990 assigned to pre-Wisconsinan non-glacial intervals possibly relate to the climatic optimum of the Sangamon Interglaciation, that is substage 5e of the URI: https://id.erudit.org/iderudit/032828ar deep-sea oxygen isotope record. These sites are East Bay and Green Point on DOI: https://doi.org/10.7202/032828ar Cape Breton Island. Addington Forks and East Milford in mainland Nova Scotia. Le Bassin and Portage-du-Cap on the Iles de la Madeleine, Québec, and Woody Cove, Newfoundland. Except for Woody Cove, none of the sites records See table of contents a complete climatic cycle, and the sequence of events must be pieced together from their disparate records. The spectra, characterized by significant amounts of thermophilous taxa that are not as abundant or present in the region today, Publisher(s) are similar in general to Holocene spectra at sites immediately south of the lower Great Lakes. Comparison of the fossil spectra from five sites with Les Presses de l'Université de Montréal modern surface spectra from eastern North America yields modern analogs which, if valid, indicate that the climate in Atlantic Canada during the climatic ISSN optimum of the last interglacial interval was more continental in character and 0705-7199 (print) considerably warmer than present. -

An Organization of the Scientific Investigation of the Indian Place«Nomenclatiire of the Maritime Provinces of Canada by W
FROM THE TRANSACTIONS OF THE ROYAL SOCIETY OF CANADA THIRD SERIES—1914 VOLUME vin An Organization of the Scientific Investigation of the Indian Place«nomenclatiire of the Maritime Provinces of Canada by W. F. GANONG. M.AHBb.E OTTAWA PRINTED FOR THE ROYAL SOCIETY OF CANADA 19 14 Transactions of The Royal Society of Canada SECTION II SERIES III DECEMBER 1914 VOL. VIII An Organization of the Scientific Investigation of the Indian Place- nomenclature of the Maritime Provinces of Canada, (Fourth Paper). By W. F. GANONG, M.A., Ph.D. (Read by Title May 27, 1914.) This paper is identical in aim and method with its three pre decessors, which were published in the immediately foregoing volumes of these Transactions. In a word, I am trying to apply the principles of scientific analysis to a very interesting subject especially prone to doubt and error. The comparative method which I use, explained in the introduction to the first paper, is proving wonderfully successful in solving the problems, as this paper will further illustrate. For convenience of reference I may add that the former papers made analysis of the names Oromocto, Magaguadavic, Upsalquitch, Manan, Nepisiguit, Kouchibouguac, Anagance, Wagan, Pokiok, Penniac, Bocabec, Pentagoet-Penobscot, Pohenegamook, and Cobs- cook, and used the roots thus made available in the analysis of a good many other words, both existent and extinct, of lesser importance. Of these extinct Indian names,—indigenous to the country, ap propriate to the places, and often reducible to a highly pleasing form, —the greater number may be revived to obvious advantage when additional place-names become needed in future; and I have tried to suggest simplified and softened forms for such purpose. -

Populations, Movements and Seasonal Distribution of Mergansers
F y F Environment Canada Environnement Canada Wildlife Service Service de la Faune Population s9 movements and seasonal distribution of mergansers in northern Cape Breton Island by A. J. Erskine Canadian Wildlife Service Report Series-Number 17 Issued under the authority of the Honourable Jack Davis, P.C., M.P. Minister of the Environment John S. Tener, Director Canadian Wildlife Service ©Crown Copyrights reserved Available by mail from Information Canada, Ottawa, and at the following Information Canada bookshops: Halifax 1735 Barrington Street Montreal 1182 St. Catherine Street West Ottawa 171 Slater Street Toronto 221 Yonge Street Winnipeg 393 Portage Avenue Vancouver 657 Granville Street or through your bookseller Price $1.00 Catalogue No. CW65-8/17 Price subject to change without notice Information Canada, Ottawa, 1972 Design: Gottschalk+Ash Ltd. Cover photo by Norman R. Lightfoot Contents 7 Acknowledgements 8 Perspective 9 Abstract 9 Resume 11 Introduction 13 The study area 15 Methods 15 Assessment of merganser populations 17 Banding and associated studies 17 Results 17 Seasonal chronology within study area 20 Movements of mergansers shoivn by band recoveries 22 Merganser populations in northern Cape Breton Island 22 The Margaree River system 25 Other river systems and Lake Ainslie 26 Discussion 26 Seasonal chronology 28 Movements shown by band recoveries 28 Merganser populations 28 77ie Margaree River system 30 Other river systems and Lake Ainslie 31 The aftermath of merganser shooting 32 Literature cited 33 Appendices 5 List of tables List of appendices List of figures 1. Numbers of recoveries in 1957-69, by 1. Winter counts of mergansers, 1960-64, 1. -

East Bay Hills Wind Project Mi'kmaq Ecological Knowledge Study
East Bay Hills Wind Project Mi’kmaq Ecological Knowledge Study Prepared for: Cape Breton Hydro Inc. December 2012 – Version 1 M.E.K.S. Project Team Jason Googoo, Project Manager Dave Moore, Author and Research Craig Hodder, Author and GIS Technician Mary Ellen Googoo, MEKS Interviewer John Sylliboy, MEKS Traditionalist Prepared by: Reviewed by: ___________________ ____________________ Craig Hodder, Author Jason Googoo, Manager Executive Summary This Mi’kmaq Ecological Knowledge Study, also commonly referred to as an MEKS or a Traditional Ecological Knowledge Study (TEKS), was developed by Membertou Geomatics Solutions (MGS) on behalf of Cape Breton Hydro Inc. (CBHI) for the proposed East Bay Hills Wind Power Project. This MEKS mandate is to consider land and water areas which the proposed project will utilize, and to identify what Mi’kmaq traditional use activities have occurred, or are currently occurring within, and what Mi’kmaq ecological knowledge presently exists in regards to the area. In order to ensure accountability and ethic responsibility of this MEKS, the MEKS development has adhered to the “Mi’kmaq Ecological Knowledge Protocol”. This protocol is a document that has been established by the Assembly of Nova Scotia Mi’kmaq Chiefs, which speaks to the process, procedures and results that are expected of a MEKS. The Mi’kmaq Ecological Knowledge Study consisted of two major components: • Mi’kmaq Traditional Land and Resource Use Activities , both past and present, • A Mi’kmaq Significance Species Analysis , considering the resources that are important to Mi’kmaq use. The Mi’kmaq Traditional Land and Resource Use Activities component utilized interviews as the key source of information regarding Mi’kmaq use in the Project Site and Study Area. -

Monitoring the Bras D'or Lakes: 2009-2012
Monitoring the Bras d’Or Lakes: 2009-2012 A. Drozdowski, E. Horne and G. L. Bugden Coastal Ecosystem Sciences Division Maritimes Region Fisheries and Oceans Canada Bedford Institute of Oceanography P.O. Box 1006 Dartmouth, Nova Scotia Canada B2Y 4A2 2014 Canadian Technical Report of Fisheries and Aquatic Sciences 3087 Canadian Technical Report of Fisheries and Aquatic Sciences Technical reports contain scientific and technical information that contributes to existing knowledge but which is not normally appropriate for primary literature. Technical reports are directed primarily toward a worldwide audience and have an international distribution. No restriction is placed on subject matter and the series reflects the broad interests and policies of the Department of Fisheries and Oceans, namely, fisheries and aquatic sciences. Technical reports may be cited as full publications. The correct citation appears above the abstract of each report. Each report is abstracted in Aquatic Sciences and Fisheries Abstracts and indexed in the Department’s annual index to scientific and technical publications. Numbers 1 - 456 in this series were issued as Technical Reports of the Fisheries Research Board of Canada. Numbers 457 - 714 were issued as Department of the Environment, Fisheries and Marine Service Technical Reports. The current series name was changed with report number 925. Technical reports are produced regionally but are numbered nationally. Requests for individual reports will be filled by the issuing establishment listed on the front cover and title page. Out-of-stock reports will be supplied for a fee by commercial agents. Rapport technique canadien des sciences halieutiques et aquatiques Les rapports techniques contiennent des renseignements scientifiques et techniques qui constituent une contribution aux connaissances actuelles, mais que ne sont pas normalement appropriés pour la publication dans un journal scientifique. -
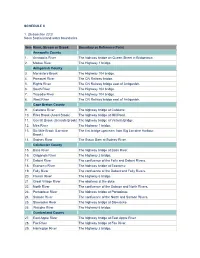
Nova Scotia Inland Water Boundaries Item River, Stream Or Brook
SCHEDULE II 1. (Subsection 2(1)) Nova Scotia inland water boundaries Item River, Stream or Brook Boundary or Reference Point Annapolis County 1. Annapolis River The highway bridge on Queen Street in Bridgetown. 2. Moose River The Highway 1 bridge. Antigonish County 3. Monastery Brook The Highway 104 bridge. 4. Pomquet River The CN Railway bridge. 5. Rights River The CN Railway bridge east of Antigonish. 6. South River The Highway 104 bridge. 7. Tracadie River The Highway 104 bridge. 8. West River The CN Railway bridge east of Antigonish. Cape Breton County 9. Catalone River The highway bridge at Catalone. 10. Fifes Brook (Aconi Brook) The highway bridge at Mill Pond. 11. Gerratt Brook (Gerards Brook) The highway bridge at Victoria Bridge. 12. Mira River The Highway 1 bridge. 13. Six Mile Brook (Lorraine The first bridge upstream from Big Lorraine Harbour. Brook) 14. Sydney River The Sysco Dam at Sydney River. Colchester County 15. Bass River The highway bridge at Bass River. 16. Chiganois River The Highway 2 bridge. 17. Debert River The confluence of the Folly and Debert Rivers. 18. Economy River The highway bridge at Economy. 19. Folly River The confluence of the Debert and Folly Rivers. 20. French River The Highway 6 bridge. 21. Great Village River The aboiteau at the dyke. 22. North River The confluence of the Salmon and North Rivers. 23. Portapique River The highway bridge at Portapique. 24. Salmon River The confluence of the North and Salmon Rivers. 25. Stewiacke River The highway bridge at Stewiacke. 26. Waughs River The Highway 6 bridge. -

Doers & Dreamers Travel Guide
Getting Around The travel times provided are approximate and have been calculated using Google Maps. Depending on the route between the destination points, Google considers both highway and secondary roads in the calculation. Please be aware that your travel time will be affected by other factors, such as side trips to attractions and activities in the region. 2020 DOERS & DREAMERS TRAVEL GUIDE Halifax International Maine to Amherst Digby Halifax North Sydney Pictou Yarmouth Airport Nova Scotia Advocate Harbour 2hr05 96km 5hr00 427km 3hr00 227km 5hr45 444km 2hr40 200km 6hr10 511km 2hr40 197km Amherst — — 4hr00 397km 2hr00 197km 4hr15 411km 2hr00 140km 5hr05 496km 1hr40 166km Annapolis Royal 3hr45 365km 0hr30 37km 2hr15 203km 6hr10 576km 3hr30 333km 1hr35 136km 2hr15 214km 2020 DOERS & DREAMERS TRAVEL GUIDE | 1-800-565-0000 2020 DOERS & DREAMERS TRAVEL Antigonish 2hr10 217km 4hr05 415km 2hr15 212km 2hr20 196km 0hr55 76km 5hr15 496km 1hr50 175km Aylesford 3hr00 300km 1hr10 100km 1hr30 130km 5hr25 510km 2hr40 268km 2hr10 198km 1hr25 141km Baddeck 3hr40 355km 5hr45 552km 3hr45 350km 0hr40 58km 2hr25 214km 6hr45 651km 3hr20 312km Bridgewater 3hr00 279km 2hr05 140km 1hr15 102km 5h20 489km 2hr45 247km 2hr20 204km 1hr20 115km Cape North 5hr45 490km 7hr45 688km 5hr45 485km 2hr20 140km 4hr25 349km 8hr45 768km 5hr20 447km Chéticamp 4hr40 400km 6hr35 595km 4hr40 395km 2hr00 145km 3hr20 257km 7hr50 678km 4hr25 364km Clark's Harbour 4hr45 437km 2hr10 180km 3hr10 262km 7hr15 649km 4hr35 405km 1hr05 81km 3hr25 280km Digby 4hr00 397km —— 2hr30 230km 6hr20 608km 3hr45 368km 1hr10 105km 2hr30 239km Guysborough 3hr00 279km 4hr55 477km 3hr00 274km 2hr30 199km 1hr40 138km 6hr05 557km 2hr45 235km Halifax 2hr00 197km 2hr30 230km —— 4hr20 408km 1hr45 165km 3hr20 304km 0hr31 39km Halifax Int. -

Bras D'or Lakes Scenic Drive Bras D'or Lakes Scenic Drive
BrasBras d’Ord’Or LakesLakes ScenicScenic DriveDrive Reflections on the beautiful Bras d’Or Visitor Information Finest Sailing in the World Centres WELCOME TO CAPE BRETON’S rolling heartland, where the highlands meet Baddeck E15, 295-1911 the lowlands along the shores of the island’s beautiful inland sea— Little Narrows, 756-2413 the Bras d’Or Lakes. The Bras d’Or Lakes Scenic Drive circles the lake Louisbourg E17, 733-2720 along shoreline roads that offer an ever-changing panorama Margaree Forks D14, 248-2803 North Sydney D16, 794-7719 of woodlands, farms and villages, and are ideal for walking, biking and bird- ≈ Port Hastings F13, 625-4201 watching. The region is a major nesting area for bald eagles, and these impressive Port Hood E13, 787-2521 birds can often be seen soaring aloft or perched on shoreline trees. St. Peter’s F14, 535-2185 There’s something new around every corner along this route. Experience the daily Sydney River E16, 539-9876 life of the early Scottish settlers at the Nova Scotia Highland Village Museum. Most Visitor Information Centres At Marble Mountain Museum you can learn about marble quarrying in the late are open mid-May to mid-October. Call the above numbers or 1800s, and the Orangedale Railway Station Museum offers a special look at late 1-800-565-0000. th 19 -century trains and train travel. ≈ Provides information for Known for its gentle, fog-free waters, beautiful anchorages, and hundreds of all of Nova Scotia coves and islands, the lakes are an international cruising destination, attracting cbisland.com hundreds of boating enthusiasts every year.