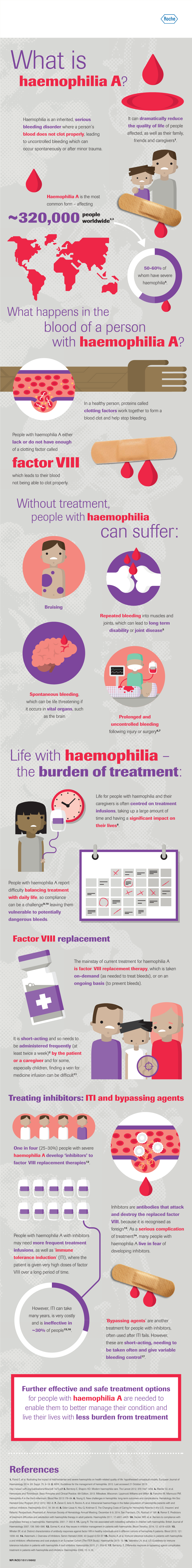
Haemophilia a Is the Most Common Form – Affecting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PATHOLOGY RESIDENT HEMATOLOGY ROTATION (North Florida/South Georgia Veterans Health Care System): Rotation Director: William L
PATHOLOGY RESIDENT HEMATOLOGY ROTATION (North Florida/South Georgia Veterans Health Care System): Rotation Director: William L. Clapp, M.D., Chief, Hematology Section, Gainesville VAMC; Consultants: Neil S. Harris, M.D., Director, Laboratory Hematology/Coagulation, University of Florida and Shands Hospital and Raul C. Braylan, M.D., Director, Hematopathology, University of Florida and Shands Hospital 1. Description of the Rotation: In this rotation, the resident will gain experience in laboratory hematology, which will include (1) peripheral blood studies to evaluate a variety of hematologic disorders, including anemias, lymphoproliferative and myeloproliferative disorders and leukemias. The emphasis on a multidisciplinary approach to diagnose hematologic disorders (including correlation of the peripheral blood studies with bone marrow and lymph node studies) provides an opportunity for the resident to also gain additional experience in (2) traditional histopathology, (3) immunohistochemistry, (4) electron microscopy, (5) protein electrophoresis, (6) flow cytometry, (7) cytogenetics and (8) molecular genetics which may be performed on the peripheral blood, bone marrow or lymph nodes of patients. The residents will acquire valuable experience by independently performing some bone marrow procedures. In addition, the resident will gain experience in coagulation testing. The residents will become familiar with the instrumentation in the hematology laboratory, including the operating principles and trouble-shooting (medical knowledge). The availability of assembled case study sets and reading materials (medical knowledge) will enhance the resident’s experience. Participation in CAP surveys, continuing education and hematology conferences is a component of the rotation (practice-based learning). Management issues and computer applications will be discussed (practice-based learning). As appropriate to the individual case or consultation under review, the ethical, socioeconomic, medicolegal and cost-containment issues will be reviewed and discussed. -

Section 8: Hematology CHAPTER 47: ANEMIA
Section 8: Hematology CHAPTER 47: ANEMIA Q.1. A 56-year-old man presents with symptoms of severe dyspnea on exertion and fatigue. His laboratory values are as follows: Hemoglobin 6.0 g/dL (normal: 12–15 g/dL) Hematocrit 18% (normal: 36%–46%) RBC count 2 million/L (normal: 4–5.2 million/L) Reticulocyte count 3% (normal: 0.5%–1.5%) Which of the following caused this man’s anemia? A. Decreased red cell production B. Increased red cell destruction C. Acute blood loss (hemorrhage) D. There is insufficient information to make a determination Answer: A. This man presents with anemia and an elevated reticulocyte count which seems to suggest a hemolytic process. His reticulocyte count, however, has not been corrected for the degree of anemia he displays. This can be done by calculating his corrected reticulocyte count ([3% × (18%/45%)] = 1.2%), which is less than 2 and thus suggestive of a hypoproliferative process (decreased red cell production). Q.2. A 25-year-old man with pancytopenia undergoes bone marrow aspiration and biopsy, which reveals profound hypocellularity and virtual absence of hematopoietic cells. Cytogenetic analysis of the bone marrow does not reveal any abnormalities. Despite red blood cell and platelet transfusions, his pancytopenia worsens. Histocompatibility testing of his only sister fails to reveal a match. What would be the most appropriate course of therapy? A. Antithymocyte globulin, cyclosporine, and prednisone B. Prednisone alone C. Supportive therapy with chronic blood and platelet transfusions only D. Methotrexate and prednisone E. Bone marrow transplant Answer: A. Although supportive care with transfusions is necessary for treating this patient with aplastic anemia, most cases are not self-limited. -

Acquired Hemophilia A: Pathogenesis and Treatment
Bleeding disorders Acquired hemophilia A: pathogenesis and treatment P.W. Collins ABSTRACT Arthur Bloom Haemophilia Centre, Acquired hemophilia A is an autoimmune disease caused by an inhibitory antibody to factor VIII. The School of Medicine, severity of bleeding varies but patients remain at risk of life-threatening bleeding until the inhibitor Cardiff University, Heath Park, has been eradicated. The cornerstones of management are rapid and accurate diagnosis, control of Cardiff, UK bleeding, investigation for an underlying cause, and eradication of the inhibitor by immunosuppres - sion. Patients should be managed jointly with a specialist center even if they present without signifi - cant bleeding. Despite an extensive literature, few controlled data are available and management Hematology Education: guidelines are based on expert opinion. Recombinant factor VIIa and activated prothrombin complex the education program for the concentrate are equally efficacious for treating bleeds and both are superior to factor VIII or desmo - annual congress of the European pressin. Immunosuppression should be started as soon as the diagnosis is made. Commonly used reg - Hematology Association imens are steroids alone or combined with cytotoxic agents. Rituximab is being used more commonly but current evidence does not suggest that it improves outcomes or reduces side effects. 2012;6:65-72 Introduction Pathogenesis Acquired hemophilia A (AHA) is a bleed - AHA is associated with autoimmune dis - ing disorder caused by polyclonal IgG1 and eases, such as rheumatoid arthritis, polymyal - IgG4 autoantibodies to the factor VIII ( FVIII ) gia rheumatic, and systemic lupus erythe - A2 and C2 domain. Morbidity and mortality matosis; malignancy; pregnancy and dermato - are high secondary to age, underlying dis - logical disorders, such as pemphigoid. -

Outcomes of Patients with Thrombocytopenia Evaluated at Hematology Subspecialty Clinics
Henry Ford Health System Henry Ford Health System Scholarly Commons Hematology Oncology Articles Hematology-Oncology 2-11-2021 Outcomes of patients with thrombocytopenia evaluated at hematology subspecialty clinics Zaid H. Abdel Rahman Kevin C. Miller H Jabbour Yaser Alkhatib Vijayalakshmi Donthireddy Follow this and additional works at: https://scholarlycommons.henryford.com/ hematologyoncology_articles Hematol Oncol Stem Cell Ther xxx (xxxx) xxx Available at www.sciencedirect.com ScienceDirect journal homepage: www.elsevier.com/locate/hemonc Outcomes of patients with thrombocytopenia evaluated at hematology subspecialty clinics Zaid H. Abdel Rahman a,*, Kevin C. Miller b, Hiba Jabbour c, Yaser Alkhatib c, Vijaya Donthireddy c a Division of Hematology and Medical Oncology, Mayo Clinic, Jacksonville, FL, USA b Department of Medicine, Massachusetts General Hospital, Boston, MA, USA c Division of Hematology and Medical Oncology, Henry Ford Hospital, Detroit, MI, USA Received 6 October 2020; received in revised form 9 December 2020; accepted 15 January 2021 KEYWORDS Abstract Hematology; Background: Thrombocytopenia is a frequently encountered laboratory abnormality and a Malignancy; common reason for hematology referrals. Workup for thrombocytopenia is not standardized Platelets; and frequently does not follow an evidence-based algorithm. We conducted a systematic anal- Referrals; Thrombocytopenia ysis to evaluate the laboratory testing and outcomes of patients evaluated for thrombocytope- nia at hematology clinics in a tertiary referral center between 2013 and 2016. Patient and methods: We performed a comprehensive chart review for patients evaluated for thrombocytopenia during the study period. Patients were followed for 1 year from the initial hematology evaluation and assessed for the development of a hematologic malignancy, rheumatologic, or infectious diseases among other clinical outcomes. -

Alpha Thalassemia Trait
Alpha Thalassemia Trait Alpha Thalassemia Trait Produced by St. Jude Children’s Research Hospital, Departments of Hematology, Patient Education, 1 and Biomedical Communications. Funds were provided by St. Jude Children’s Research Hospital, ALSAC, and a grant from the Plough Foundation. This document is not intended to replace counseling by a trained health care professional or genetic counselor. Our aim is to promote active participation in your care and treatment by providing information and education. Questions about individual health concerns or specific treatment options should be discussed with your doctor. For general information on sickle cell disease and other blood disorders, please visit our Web site at www.stjude.org/sicklecell. Copyright © 2009 St. Jude Children’s Research Hospital Alpha thalassemia trait All red blood cells contain hemoglobin (HEE muh glow bin), which carries oxygen from your lungs to all parts of your body. Alpha thalassemia (thal uh SEE mee uh) trait is a condition that affects the amount of hemo- globin in the red blood cells. • Adult hemoglobin (hemoglobin A) is made of alpha and beta globins. • Normally, people have 4 genes for alpha globin with 2 genes on each chromosome (aa/aa). People with alpha thalassemia trait only have 2 genes for alpha globin, so their bodies make slightly less hemoglobin than normal. This trait was passed on from their parents, like hair color or eye color. A trait is different from a disease 2 Alpha thalassemia trait is not a disease. Normally, a trait will not make you sick. Parents who have alpha thalassemia trait can pass it on to their children. -

Hematology/Oncology
Hematology/Oncology Description: The pediatric hematology-oncology division sees a wide spectrum of pediatric disease including but not limited to leukemia, hemophilia, solid tumors, ITP, and other blood dyscrasias. The pediatric resident is expected to be involved in the work-up and on-going management of all patient presenting to the hem-onc service. Note: The goals and objectives described in detail below are not meant to be completed in a single one month block rotation but are meant to be cumulative, culminating in a thorough and complete Pediatric Hem-Onc experience at the end of residency. Primary Goals for this Rotation GOAL: Prevention, Counseling and Screening. Understand the role of the pediatrician in preventing hematologic or oncologic conditions, and in counseling and screening individuals at risk for these diseases. Provide routine preventive counseling about hematology to all patients and families, addressing: 1. Adequate diet and iron intake to prevent iron deficiency 2. Signs and symptoms of malignant disease Provide preventive counseling to parents and patients with specific hematology/oncology conditions, addressing: 1. In a child with a sickle hemoglobinopathy, the importance of antibiotic prophylaxis, pneumococcal and routine immunizations, folic acid supplementation, and urgent need for evaluation for fever 2. Risk of infections related to transfusion of blood or blood products, and alternatives to routine transfusion (i.e., direct donation, irradiation, freezing, filtration) 3. Expected course of common childhood malignancies, with good and bad prognosticators 4. Support groups and information available for children with cancer Provide regular hematology/oncology screening for patients: 1. Screen for hemoglobinopathies in the newborn period. -

Autoimmune Hemolytic Anemia in COVID-19 Patients, the « Transmissible » Direct Coombs Test
J H C R JOURNAL OF HEMATOLOGY 2640-2823 AND CLINICAL RESEARCH Research Article More Information *Address for Correspondence: Alice Brochier, Hematology Department of Laboratory Medicine, Autoimmune hemolytic anemia in Saint-Luc University Hospital, Avenue Hippocrate 10, 1200 Brussels, Belgium, Tel: +322764 6814; COVID-19 patients, the « transmissible » Email: [email protected]; Véronique Deneys, Hematology Department of Laboratory Medicine, Saint-Luc University direct Coombs test Hospital, Avenue Hippocrate 10, 1200 Brussels, Belgium, Email: [email protected] Alice Brochier1*, Julien Cabo1, Claudine Guerrieri1, Leïla Belkhir2, Submitted: March 24, 2021 3 1 Pierre-François Laterre and Véronique Deneys * Approved: April 06, 2021 Published: April 07, 2021 1Hematology Department of Laboratory Medicine, Saint-Luc University Hospital, Brussels, Belgium 2Department of Internal Medicine and Infectious Diseases, Saint-Luc University Hospital, Brussels, How to cite this article: Brochier A, Cabo J, Guerrieri C, Belkhir L, Laterre PF, Deneys V. Belgium Autoimmune hemolytic anemia in COVID-19 3 Department of Intensive Care Medicine, Saint-Luc University Hospital, Brussels, Belgium patients, the « transmissible » direct Coombs test. J Hematol Clin Res. 2021; 5: 004-008. Abstract DOI: 10.29328/journal.jhcr.1001016 Copyright: © 2021 Brochier A, et al. This Background: Like other viruses, the SARS-CoV-2 (severe acute respiratory syndrome is an open access article distributed under coronavirus 2) appears to be responsible for several autoimmune complications. The occurrence the Creative Commons Attribution License, of autoimmune hemolytic anemia has been described in several case reports. This AIHA was also which permits unrestricted use, distribution, noticeable by the important number of blood transfusions required for COVID-19 (coronavirus and reproduction in any medium, provided the disease 2019) patients. -

1-Cm Interval on Chromosome 15Q15.1-15.3
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Am. J. Hum. Genet. 62:1062–1069, 1998 Localization of the Gene for Congenital Dyserythropoietic Anemia Type I to a !1-cM Interval on Chromosome 15q15.1-15.3 Hannah Tamary,1,2 Lea Shalmon,2 Hanna Shalev,3 Albudar Halil,3 Dina Dobrushin,2 Noga Ashkenazi,2 Meira Zoldan,1 Peretz Resnitzky,4 Michael Korostishevsky,5 Batsheva Bonne-Tamir,5 and Rina Zaizov,1,2 1Pediatric Hematology Oncology Center, Schneider Children’s Medical Center of Israel, and 2Pediatric Hematology Oncology Research Laboratory, The Felsenstein Medical Research Center, Beilinson Campus, Petah Tiqva, Israel; 3Division of Pediatrics, Soroka Medical Center, Beersheba, Israel; 4Internal Medicine B, and the Efrati Research Institute for Blood Cells and Cytology, Kaplan Hospital, Rehovot, Israel; and 5Department of Human Genetics; Sackler School of Medicine, Tel Aviv University, Tel Aviv Summary Introduction Congenital dyserythropoietic anemias (CDA) are a rare Congenital dyserythropoietic anemias (CDA) are a rare group of red-blood-cell disorders of unknown etiology group of red-blood-cell disorders of unknown etiology that are characterized by ineffective erythropoiesis, pa- that are characterized by ineffective erythropoiesis, pa- thognomonic cytopathology of the nucleated red blood thognomonic cytopathology of the nucleated red blood cells in the bone marrow, and secondary hemochro- cells in the bone marrow, and secondary hemochro- matosis. In CDA type I, bone-marrow electron micros- matosis. In 1968, Heimpel and Wendt classified these copy reveals characteristic findings in erythroid precur- disorders into three types, which were later confirmed sors, including spongy heterochromatin and enlarged by electron microscopy and serological findings (Alter nuclear pores. -

Emergency Care for Patients with Hemophilia
Eme rgency Ca re for Patients with Hemophilia An instruction al ma nual for Medic al Profession als Third Edition Written by the Nursing G roup of Hemophilia Region VI Editors: Karen Wulff , R.N.; Susan Z appa R.N., C. P.N., C. P.O.N.; Mack Womack , R.N. Determine if the patient has: Hemophilia A or factor VIII (8) deficiency Hemophilia B or factor IX (9) deficiency Head inju ry (pg. 4) Always t reat immediately with a major factor dose Mucous membrane bleeds (pg. 12) Treat with a routine factor dose and anti-fibrinolytics Slings, splints, immobilizers p. r.n. for joint bleeds Joint bleeds (pg. 6) Treat an early onset bleed with a routine factor dose. Treat an advanced joint bleed with Avoid intramuscular a major factor dose. injections due to the possibility of causing a muscle bleed Abdominal bleeds (pg. 10), Trauma (pg. 23) Crutches Treat with a major factor dose hip, knee, ankle bleeds Minor cuts / bruises no t reatment Ice pack / Ace wrap joint bleeds Factor Replacement Doses TREATMENT FOR MINOR BLEEDS Minor bleeds include: Nose (epistaxis), Mouth (including gums) Joints (hemarthroses), Abrasions and superficial lacerations Hemophilia A or Factor VIII (8) Recombinant Factor VIII concentrate Severe to moderate deficiency: Dosage 20 - 30 units per kilogram Hemophilia A or Factor VIII (8) DDAVP responsive: DDAVP dosage: 0.3 micro- Mild deficiency: grams per kilogram (Maximim dose: 20 micrograms) DDAVP non-responsive: Recombinant Factor VIII concentrate. Dosage 20 - 30 units per kilogram Hemophilia B or Factor IX (9) deficiency Recombinant -

Hematological Pathology
FREQUENTLY ASKED QUESTIONS – 2019/2020 Hematological Pathology Specialty/Field Question 1. A) What are some strengths about your specialty? What draws and keeps people in your specialty? Hematological Pathology is a specialty within laboratory medicine that integrates clinical consultation and diagnosis with quality laboratory management and practice. We provide consultations to hematology, oncology, internal medicine, anesthesiology, critical care, surgery, and organ/tissue transplant specialists in the diagnosis and investigation of hematological disorders, coagulation disorders (bleeding and thrombosis), transfusion medicine and blood product utilization, histocompatibility/immunology, and molecular pathology. In other words, we don’t just look at glass slides under the microscope! We enjoy having a rich variety of clinical encounters with other clinicians and specialists. The resulting breadth and depth of hematopathology practice is the most attractive and exciting aspect of our daily work. B) What are some common complaints your specialty? There are not enough of us, so we all have a lot to do! The silver lining though is that there are positions available across the country for graduating residents. Alumni from the Edmonton program have worked/are working in Victoria, Vancouver, Edmonton, Toronto, Kingston, Ottawa, and Halifax! Some have even pursued additional fellowship training in the U.S. before beginning their staff positions. Instead of having traditional contact with patients through histories and physical exams in the hospital or outpatient clinics, we encounter them through bone marrow biopsies, under the microscope during blood film and bone marrow reporting, and discussions at coagulation and transfusion medicine rounds. Since we do not see or admit patients in the traditional sense, this also means we do not have to worry about things like lack of clinic or operating room time or space, or the lack of hospital beds to admit patients, etc. -

Arizona Hemoglobin Bart's Fact Sheet for Health Care Providers
Arizona Hemoglobin Bart’s Fact Sheet for Health Care Providers Hemoglobin Barts Your patient has been found on the Arizona Newborn Genetic Screen to have a hemoglobin electrophoresis pattern consistent with "FA Bart’s". The acronym stands for the hemoglobin species present in the baby's blood in descending order of prevalence. The F designates fetal hemoglobin (a2 y2), A denotes hemoglobin A (a2ß2) and Bart’s represents hemoglobin Bart's, a tetramer of y-globin molecules (y4). Hemoglobin Barts (y4) appears in the newborn when one or more of the 4 human a-globin genes are missing. The relative over abundance of y-globin molecules leads to y4 production and the diagnosis of Hemoglobin Barts. Alpha thalassemia is caused by deletions of the alpha globin genes on chromosome 16. Normal individuals have 4 copies of the gene with 2 on each chromosome. It is possible to lose 1 to 4 of these genes. The presence of hemoglobin Bart’s on newborn screen usually suggests that the infant is missing at least 1 alpha gene. The silent carrier: One deleted Alpha Gene Neonates and children with three functional alpha genes have a complete or nearly completely silent phenotype. The red cell indices are normal and remain so for life. When only one a gene is non- functional, the hemoglobin Barts percentage is usually 1-2% in the newborn, and is not detectable when the fetal hemoglobin synthesis stops at 6 months of age. As the newborn matures, the red cells can rarely exhibit a reduced MCV, MCH, but will show normal HBA2 and F levels if the hemoglobin electrophoresis is repeated. -

Color Atlas of Hematology
i ii iii Color Atlas of Hematology Practical Microscopic and Clinical Diagnosis Harald Theml, M.D. Professor, Private Practice Hematology/Oncology Munich, Germany Heinz Diem, M.D. Klinikum Grosshadern Institute of Clinical Chemistry Munich, Germany Torsten Haferlach, M.D. Professor, Klinikum Grosshadern Laboratory for Leukemia Diagnostics Munich, Germany 2nd revised edition 262 color illustrations 32 tables Thieme Stuttgart · New York iv Library of Congress Cataloging-in-Publica- Important note: Medicine is an ever- tion Data is available from the publisher changing science undergoing continual development. Research and clinical ex- perience are continually expanding our knowledge, in particular our knowledge of This book is an authorized revised proper treatment and drug therapy. Insofar translation of the 5th German edition as this book mentions any dosage or appli- published and copyrighted 2002 by cation, readers may rest assured that the Thieme Verlag, Stuttgart, Germany. authors, editors, and publishers have made Title of the German edition: every effort to ensure that such references Taschenatlas der Hämatologie are in accordance with the state of knowl- edge at the time of production of the book. Translator: Ursula Peter-Czichi PhD, Nevertheless, this does not involve, imply, Atlanta, GA, USA or express any guarantee or responsibility on the part of the publishers in respect to any dosage instructions and forms of appli- 1st German edition 1983 cations stated in the book. Every user is re- 2nd German edition 1986 quested to examine carefully the manu- 3rd German edition 1991 facturers’ leaflets accompanying each drug 4th German edition 1998 and to check, if necessary in consultation 5th German edition 2002 with a physician or specialist, whether the 1st English edition 1985 dosage schedules mentioned therein or the 1st French edition 1985 contraindications stated by the manufac- 2nd French edition 2000 turers differ from the statements made in 1st Indonesion edition 1989 the present book.