Management of Immune Thrombocytopenia (ITP) a POCKET GUIDE for the CLINICIAN NOVEMBER 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protein S Deficiency Presenting with Hemorrhage in a Term Neonate
: Curre re nt a R C e Ayari et al., Health Care Current Reviews 2018, 6:1 h v t i l e a w DOI: 10.4172/2375-4273.1000219 e s H Health Care: Current Reviews ISSN: 2375-4273 Review Article Open Access Protein S Deficiency Presenting with Hemorrhage in a Term Neonate Fairouz Ayari*, Takoua Bensmail, Essid Latifa, Wiem Barbaria and Samia Kacem Neonatology Intensive Care Unit of the Maternity and Neonatology Center, Tunis, Tunisia Abstract Unexplained bleeding symptoms in otherwise healthy full-term usually present a diagnostic challenge for treating physicians requiring prompt and accurate laboratory investigations to ensure appropriate treatment and possibly avoid long-term morbidity. We report a case of a term neonate with severe protein S deficiency manifested by systemic hemorrhage and multiple organ failure at 9 days of age. We review how protein S influences the coagulation and the fibrinolytic pathways, discussing therapeutic approaches of neonates with purpura fulminans. Keywords: Protein S deficiency; Blood sample; Thrombophilic dis- resuscitation with 20 ml/kg bodyweight (BW) saline solution and, after order blood sampling, intravenous administration of 10 mg vitamin K, 20 ml/kg BW fresh frozen plasma, 20 ml/kg BW packed red blood cells Introduction (5 transfusion cycles), 20 mg/kg BW Phenobarbital and vasoactive Protein S (PS) is an antithrombotic plasma protein that acts mainly drugs. Cerebral ultrasound revealed intraventricular haemorrhage, as a cofactor of activated protein C (APC) anticoagulant activity in the abdominal ultrasound showed splenic hemorrhage and cardiac degradation of factor Va and activated factor VIII [1]. PS circulates in ultrasound showed a floating intracardiac thrombus. -

PATHOLOGY RESIDENT HEMATOLOGY ROTATION (North Florida/South Georgia Veterans Health Care System): Rotation Director: William L
PATHOLOGY RESIDENT HEMATOLOGY ROTATION (North Florida/South Georgia Veterans Health Care System): Rotation Director: William L. Clapp, M.D., Chief, Hematology Section, Gainesville VAMC; Consultants: Neil S. Harris, M.D., Director, Laboratory Hematology/Coagulation, University of Florida and Shands Hospital and Raul C. Braylan, M.D., Director, Hematopathology, University of Florida and Shands Hospital 1. Description of the Rotation: In this rotation, the resident will gain experience in laboratory hematology, which will include (1) peripheral blood studies to evaluate a variety of hematologic disorders, including anemias, lymphoproliferative and myeloproliferative disorders and leukemias. The emphasis on a multidisciplinary approach to diagnose hematologic disorders (including correlation of the peripheral blood studies with bone marrow and lymph node studies) provides an opportunity for the resident to also gain additional experience in (2) traditional histopathology, (3) immunohistochemistry, (4) electron microscopy, (5) protein electrophoresis, (6) flow cytometry, (7) cytogenetics and (8) molecular genetics which may be performed on the peripheral blood, bone marrow or lymph nodes of patients. The residents will acquire valuable experience by independently performing some bone marrow procedures. In addition, the resident will gain experience in coagulation testing. The residents will become familiar with the instrumentation in the hematology laboratory, including the operating principles and trouble-shooting (medical knowledge). The availability of assembled case study sets and reading materials (medical knowledge) will enhance the resident’s experience. Participation in CAP surveys, continuing education and hematology conferences is a component of the rotation (practice-based learning). Management issues and computer applications will be discussed (practice-based learning). As appropriate to the individual case or consultation under review, the ethical, socioeconomic, medicolegal and cost-containment issues will be reviewed and discussed. -

Section 8: Hematology CHAPTER 47: ANEMIA
Section 8: Hematology CHAPTER 47: ANEMIA Q.1. A 56-year-old man presents with symptoms of severe dyspnea on exertion and fatigue. His laboratory values are as follows: Hemoglobin 6.0 g/dL (normal: 12–15 g/dL) Hematocrit 18% (normal: 36%–46%) RBC count 2 million/L (normal: 4–5.2 million/L) Reticulocyte count 3% (normal: 0.5%–1.5%) Which of the following caused this man’s anemia? A. Decreased red cell production B. Increased red cell destruction C. Acute blood loss (hemorrhage) D. There is insufficient information to make a determination Answer: A. This man presents with anemia and an elevated reticulocyte count which seems to suggest a hemolytic process. His reticulocyte count, however, has not been corrected for the degree of anemia he displays. This can be done by calculating his corrected reticulocyte count ([3% × (18%/45%)] = 1.2%), which is less than 2 and thus suggestive of a hypoproliferative process (decreased red cell production). Q.2. A 25-year-old man with pancytopenia undergoes bone marrow aspiration and biopsy, which reveals profound hypocellularity and virtual absence of hematopoietic cells. Cytogenetic analysis of the bone marrow does not reveal any abnormalities. Despite red blood cell and platelet transfusions, his pancytopenia worsens. Histocompatibility testing of his only sister fails to reveal a match. What would be the most appropriate course of therapy? A. Antithymocyte globulin, cyclosporine, and prednisone B. Prednisone alone C. Supportive therapy with chronic blood and platelet transfusions only D. Methotrexate and prednisone E. Bone marrow transplant Answer: A. Although supportive care with transfusions is necessary for treating this patient with aplastic anemia, most cases are not self-limited. -

Acquired Hemophilia A: Pathogenesis and Treatment
Bleeding disorders Acquired hemophilia A: pathogenesis and treatment P.W. Collins ABSTRACT Arthur Bloom Haemophilia Centre, Acquired hemophilia A is an autoimmune disease caused by an inhibitory antibody to factor VIII. The School of Medicine, severity of bleeding varies but patients remain at risk of life-threatening bleeding until the inhibitor Cardiff University, Heath Park, has been eradicated. The cornerstones of management are rapid and accurate diagnosis, control of Cardiff, UK bleeding, investigation for an underlying cause, and eradication of the inhibitor by immunosuppres - sion. Patients should be managed jointly with a specialist center even if they present without signifi - cant bleeding. Despite an extensive literature, few controlled data are available and management Hematology Education: guidelines are based on expert opinion. Recombinant factor VIIa and activated prothrombin complex the education program for the concentrate are equally efficacious for treating bleeds and both are superior to factor VIII or desmo - annual congress of the European pressin. Immunosuppression should be started as soon as the diagnosis is made. Commonly used reg - Hematology Association imens are steroids alone or combined with cytotoxic agents. Rituximab is being used more commonly but current evidence does not suggest that it improves outcomes or reduces side effects. 2012;6:65-72 Introduction Pathogenesis Acquired hemophilia A (AHA) is a bleed - AHA is associated with autoimmune dis - ing disorder caused by polyclonal IgG1 and eases, such as rheumatoid arthritis, polymyal - IgG4 autoantibodies to the factor VIII ( FVIII ) gia rheumatic, and systemic lupus erythe - A2 and C2 domain. Morbidity and mortality matosis; malignancy; pregnancy and dermato - are high secondary to age, underlying dis - logical disorders, such as pemphigoid. -

081999 Disseminated Intravascular Coagulation
The New England Journal of Medicine Current Concepts Systemic activation+ of coagulation DISSEMINATED INTRAVASCULAR COAGULATION Intravascular+ Depletion of platelets+ deposition of fibrin and coagulation factors MARCEL LEVI, M.D., AND HUGO TEN CATE, M.D. Thrombosis of small+ Bleeding and midsize vessels+ ISSEMINATED intravascular coagulation is and organ failure characterized by the widespread activation Dof coagulation, which results in the intravas- Figure 1. The Mechanism of Disseminated Intravascular Coag- cular formation of fibrin and ultimately thrombotic ulation. occlusion of small and midsize vessels.1-3 Intravascu- Systemic activation of coagulation leads to widespread intra- lar coagulation can also compromise the blood sup- vascular deposition of fibrin and depletion of platelets and co- agulation factors. As a result, thrombosis of small and midsize ply to organs and, in conjunction with hemodynam- vessels may occur, contributing to organ failure, and there may ic and metabolic derangements, may contribute to be severe bleeding. the failure of multiple organs. At the same time, the use and subsequent depletion of platelets and coag- ulation proteins resulting from the ongoing coagu- lation may induce severe bleeding (Fig. 1). Bleeding may be the presenting symptom in a patient with disseminated intravascular coagulation, a factor that can complicate decisions about treatment. TABLE 1. COMMON CLINICAL CONDITIONS ASSOCIATED WITH DISSEMINATED ASSOCIATED CLINICAL CONDITIONS INTRAVASCULAR COAGULATION. AND INCIDENCE Sepsis Infectious Disease Trauma Serious tissue injury Disseminated intravascular coagulation is an ac- Head injury Fat embolism quired disorder that occurs in a wide variety of clin- Cancer ical conditions, the most important of which are listed Myeloproliferative diseases in Table 1. -

What Everyone Should Know to Stop Bleeding After an Injury
What Everyone Should Know to Stop Bleeding After an Injury THE HARTFORD CONSENSUS The Joint Committee to Increase Survival from Active Shooter and Intentional Mass Casualty Events was convened by the American College of Surgeons in response to the growing number and severity of these events. The committee met in Hartford Connecticut and has produced a number of documents with rec- ommendations. The documents represent the consensus opinion of a multi-dis- ciplinary committee involving medical groups, the military, the National Security Council, Homeland Security, the FBI, law enforcement, fire rescue, and EMS. These recommendations have become known as the Hartford Consensus. The overarching principle of the Hartford Consensus is that no one should die from uncontrolled bleeding. The Hartford Consensus recommends that all citizens learn to stop bleeding. Further information about the Hartford Consensus and bleeding control can be found on the website: Bleedingcontrol.org 2 SAVE A LIFE: What Everyone Should Know to Stop Bleeding After an Injury Authors: Peter T. Pons, MD, FACEP Lenworth Jacobs, MD, MPH, FACS Acknowledgements: The authors acknowledge the contributions of Michael Cohen and James “Brooks” Hart, CMI to the design of this manual. Some images adapted from Adam Wehrle, EMT-P and NAEMT. © 2017 American College of Surgeons CONTENTS SECTION 1 3 ■ Introduction ■ Primary Principles of Trauma Care Response ■ The ABCs of Bleeding SECTION 2 5 ■ Ensure Your Own Safety SECTION 3 6 ■ A – Alert – call 9-1-1 SECTION 4 7 ■ B – Bleeding – find the bleeding injury SECTION 5 9 ■ C – Compress – apply pressure to stop the bleeding by: ■ Covering the wound with a clean cloth and applying pressure by pushing directly on it with both hands, OR ■Using a tourniquet, OR ■ Packing (stuff) the wound with gauze or a clean cloth and then applying pressure with both hands SECTION 6 13 ■ Summary 2 SECTION 1: INTRODUCTION Welcome to the Stop the Bleed: Bleeding Control for the Injured information booklet. -
Haemophilia a Is the Most Common Form – Affecting
Haemophilia is an inherited, serious It can dramatically reduce bleeding disorder where a person’s the quality of life of people blood does not clot properly, leading affected, as well as their family, to uncontrolled bleeding which can friends and caregivers1. occur spontaneously or after minor trauma. Haemophilia A is the most common form – affecting 50-60% of whom have severe haemophilia4. blood of a person In a healthy person, proteins called clotting factors work together to form a blood clot and help stop bleeding. People with haemophilia A either lack or do not have enough of a clotting factor called which leads to their blood not being able to clot properly. Bruising Repeated bleeding into muscles and joints, which can lead to long term disability or joint disease5 Spontaneous bleeding, which can be life threatening if it occurs in vital organs, such as the brain Prolonged and uncontrolled bleeding following injury or surgery6,7 Life for people with haemophilia and their caregivers is often centred on treatment infusions, taking up a large amount of time and having a significant impact on their lives8. People with haemophilia A report difficulty balancing treatment with daily life, so compliance can be a challenge9,10 leaving them vulnerable to potentially dangerous bleeds. The mainstay of current treatment for haemophilia A is factor VIII replacement therapy, which is taken on-demand (as needed to treat bleeds), or on an ongoing basis (to prevent bleeds). It is short-acting and so needs to be administered frequently (at least twice a week)2 by the patient or a caregiver and for some, especially children, finding a vein for medicine infusion can be difficult11. -

Outcomes of Patients with Thrombocytopenia Evaluated at Hematology Subspecialty Clinics
Henry Ford Health System Henry Ford Health System Scholarly Commons Hematology Oncology Articles Hematology-Oncology 2-11-2021 Outcomes of patients with thrombocytopenia evaluated at hematology subspecialty clinics Zaid H. Abdel Rahman Kevin C. Miller H Jabbour Yaser Alkhatib Vijayalakshmi Donthireddy Follow this and additional works at: https://scholarlycommons.henryford.com/ hematologyoncology_articles Hematol Oncol Stem Cell Ther xxx (xxxx) xxx Available at www.sciencedirect.com ScienceDirect journal homepage: www.elsevier.com/locate/hemonc Outcomes of patients with thrombocytopenia evaluated at hematology subspecialty clinics Zaid H. Abdel Rahman a,*, Kevin C. Miller b, Hiba Jabbour c, Yaser Alkhatib c, Vijaya Donthireddy c a Division of Hematology and Medical Oncology, Mayo Clinic, Jacksonville, FL, USA b Department of Medicine, Massachusetts General Hospital, Boston, MA, USA c Division of Hematology and Medical Oncology, Henry Ford Hospital, Detroit, MI, USA Received 6 October 2020; received in revised form 9 December 2020; accepted 15 January 2021 KEYWORDS Abstract Hematology; Background: Thrombocytopenia is a frequently encountered laboratory abnormality and a Malignancy; common reason for hematology referrals. Workup for thrombocytopenia is not standardized Platelets; and frequently does not follow an evidence-based algorithm. We conducted a systematic anal- Referrals; Thrombocytopenia ysis to evaluate the laboratory testing and outcomes of patients evaluated for thrombocytope- nia at hematology clinics in a tertiary referral center between 2013 and 2016. Patient and methods: We performed a comprehensive chart review for patients evaluated for thrombocytopenia during the study period. Patients were followed for 1 year from the initial hematology evaluation and assessed for the development of a hematologic malignancy, rheumatologic, or infectious diseases among other clinical outcomes. -

Severe Fever with Thrombocytopenia Syndrome: a Newly Discovered Emerging Infectious Disease
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector REVIEW Severe fever with thrombocytopenia syndrome: a newly discovered emerging infectious disease D. X. Li Key Laboratory for Medical Virology, National Institute for Viral Disease Control and Prevention, China CDC, Beijing, China Abstract Severe fever with thrombocytopenia syndrome (SFTS) is a newly discovered emerging infectious disease that has recently become epidemic in Asia. The causative agent of SFTS is a novel phlebovirus in the family Bunyaviridae, designated SFTS virus (SFTSV). SFTS clinically presents with high fever, thrombocytopenia, leukocytopenia, gastrointestinal disorders, and multi-organ dysfunction, with a high viral load and a high case- fatality rate. In human infection, SFTSV targets microphages, replicates in the spleen of infected mice, and causes thrombocytopenia and a cytokine storm. The tick disseminates virus to humans and animals, forming a special transmission model in nature. Person-to-person transmission though direct contact with patient blood has been frequently reported. Measurements of viral RNA and antibodies have been established for diagnosis, but vaccines and specific therapeutics are not available so far. Clinical Microbiology and Infection © 2015 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved. Keywords: Clinical, epidemiology, SFTS virus, SFTS, virology Article published online: 11 March 2015 Virology D.X. Li, Key Laboratory for Medical Virology, NCHFP, RRC, National Institute for Viral Disease Control and Prevention, China CDC, Bei- jing 102206, China E-mail: [email protected] The causative agent of SFTS is SFTSV, which is a tick-borne virus in the family Bunyaviridae, genus Phlebovirus. -

Alpha Thalassemia Trait
Alpha Thalassemia Trait Alpha Thalassemia Trait Produced by St. Jude Children’s Research Hospital, Departments of Hematology, Patient Education, 1 and Biomedical Communications. Funds were provided by St. Jude Children’s Research Hospital, ALSAC, and a grant from the Plough Foundation. This document is not intended to replace counseling by a trained health care professional or genetic counselor. Our aim is to promote active participation in your care and treatment by providing information and education. Questions about individual health concerns or specific treatment options should be discussed with your doctor. For general information on sickle cell disease and other blood disorders, please visit our Web site at www.stjude.org/sicklecell. Copyright © 2009 St. Jude Children’s Research Hospital Alpha thalassemia trait All red blood cells contain hemoglobin (HEE muh glow bin), which carries oxygen from your lungs to all parts of your body. Alpha thalassemia (thal uh SEE mee uh) trait is a condition that affects the amount of hemo- globin in the red blood cells. • Adult hemoglobin (hemoglobin A) is made of alpha and beta globins. • Normally, people have 4 genes for alpha globin with 2 genes on each chromosome (aa/aa). People with alpha thalassemia trait only have 2 genes for alpha globin, so their bodies make slightly less hemoglobin than normal. This trait was passed on from their parents, like hair color or eye color. A trait is different from a disease 2 Alpha thalassemia trait is not a disease. Normally, a trait will not make you sick. Parents who have alpha thalassemia trait can pass it on to their children. -
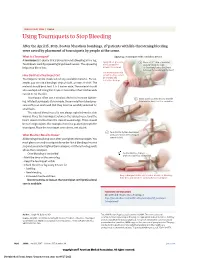
Using Tourniquets to Stop Bleeding
JAMA PATIENT PAGE | Trauma Using Tourniquets to Stop Bleeding After the April 15, 2013, Boston Marathon bombings, 27 patients with life-threatening bleeding were saved by placement of tourniquets by people at the scene. What Is a Tourniquet? Applying a tourniquet with a windlass device A tourniquet is a device that is placed around a bleeding arm or leg. Apply direct pressure 1 Place a 2-3” strip of material Tourniquets work by squeezing large blood vessels. The squeezing to the wound for about 2” from the edge helps stop blood loss. at least 15 minutes. of the wound over a long bone between the wound and the heart. Use a tourniquet only How Do I Put a Tourniquet On? when bleeding cannot be stopped and Tourniquets can be made out of any available material. For ex- is life threatening. ample, you can use a bandage, strip of cloth, or even a t-shirt. The material should be at least 2 to 3 inches wide. The material should also overlap itself. Using thin straps or material less than 2 inches wide can rip or cut the skin. Tourniquets often use a windlass device to increase tighten- 2 Insert a stick or other strong, straight ing. Inflated tourniquets (for example, those made from blood pres- item into the knot to act as a windlass. sure cuffs) can work well. But they must be carefully watched for small leaks. The injured blood vessel is not always right below the skin wound. Place the tourniquet between the injured vessel and the heart, about 2 inches from the closest wound edge. -

Hematology/Oncology
Hematology/Oncology Description: The pediatric hematology-oncology division sees a wide spectrum of pediatric disease including but not limited to leukemia, hemophilia, solid tumors, ITP, and other blood dyscrasias. The pediatric resident is expected to be involved in the work-up and on-going management of all patient presenting to the hem-onc service. Note: The goals and objectives described in detail below are not meant to be completed in a single one month block rotation but are meant to be cumulative, culminating in a thorough and complete Pediatric Hem-Onc experience at the end of residency. Primary Goals for this Rotation GOAL: Prevention, Counseling and Screening. Understand the role of the pediatrician in preventing hematologic or oncologic conditions, and in counseling and screening individuals at risk for these diseases. Provide routine preventive counseling about hematology to all patients and families, addressing: 1. Adequate diet and iron intake to prevent iron deficiency 2. Signs and symptoms of malignant disease Provide preventive counseling to parents and patients with specific hematology/oncology conditions, addressing: 1. In a child with a sickle hemoglobinopathy, the importance of antibiotic prophylaxis, pneumococcal and routine immunizations, folic acid supplementation, and urgent need for evaluation for fever 2. Risk of infections related to transfusion of blood or blood products, and alternatives to routine transfusion (i.e., direct donation, irradiation, freezing, filtration) 3. Expected course of common childhood malignancies, with good and bad prognosticators 4. Support groups and information available for children with cancer Provide regular hematology/oncology screening for patients: 1. Screen for hemoglobinopathies in the newborn period.