Emerald Ash Borer Will Affect Maryland's Eastern Shore Wetlands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Notes on Fraxinus Profunda (Oleaceae)
Nesom, G.L. 2010. Notes on Fraxinus profunda (Oleaceae). Phytoneuron 2010-32: 1–6. Mailed 10 August 2010. NOTES ON FRAXINUS PROFUNDA (OLEACEAE) Guy L. Nesom 2925 Hartwood Drive Fort Worth, TX 76109, USA www.guynesom.com ABSTRACT A taxonomic overview is provided for Fraxinus profunda –– including a nomenclatural summary with lectotypes designated for F. profunda and the synonymous F. michauxii , an updated morphological description including a comparison with F. pennsylvanica , and a county-level distribution map. Geographically disjunct records for F. profunda in distinctly inland localities (Mississippi and Alabama) are documented; far-inland records from Tennessee and North Carolina were based on collections of F. biltmoreana . KEY WORDS : Fraxinus profunda , F. michauxii , F. pennsylvanica , Oleaceae Fraxinus profunda occurs primarily along the Atlantic and Gulf coasts into peninsular Florida and in drainages of the Mississippi River and in the Ohio River basin (Little 1977; McCormac et al. 1995). At the northwestern corner of its range, it occurs in bottomlands of the Kankakee River (vPlants 2010), an Illinois/Mississippi River tributary. Limits of the northern range of the species (Michigan, Ontario) have recently been documented in detail (McCormac et al. 1995; Waldron et al. 1996). The trees consistently grow in river swamps and floodplains, especially those seasonally inundated, freshwater tidal wetlands, commonly with bald cypress, swamp cottonwood, and water tupelo. In Illinois, Indiana, Ohio, and northward, they often are found in wet woods and swampy depressions in upland woods as well as till plains and clay lake plains of post-glacial lake beds. Based on the map from Little (1977), Harms (1990) noted that the range of Fraxinus profunda is “quite discontinuous,” but addition of recent records shows a more continuous range (Fig. -

Fraxinus Spp. Family: Oleaceae American Ash
Fraxinus spp. Family: Oleaceae American Ash Ash ( Fraxinus sp.) is composed of 40 to 70 species, with 21 in Central and North America and 50 species in Eurasia. All species look alike microscopically. The name fraxinus is the classical Latin name for ash. Fraxinus americana*- American White Ash, Biltmore Ash, Biltmore White Ash, Canadian Ash, Cane Ash, Green Ash, Ground Ash, Mountain Ash, Quebec Ash, Red Ash, Smallseed White Ash, White Ash , White River Ash, White Southern Ash Fraxinus anomala-Dwarf Ash, Singleleaf Ash Fraxinus berlandierana-Berlandier Ash , Mexican Ash Fraxinus caroliniana-Carolina Ash , Florida Ash, Pop Ash, Swamp Ash, Water Ash Fraxinus cuspidata-Flowering Ash, Fragrant Ash Fraxinus dipetala-California Flwoering Ash, California Shrub Ash, Foothill Ash, Flowering Ash, Fringe- flowering Ash, Mountain Ash, Two-petal Ash Fraxinus gooddingii-Goodding Ash Fraxinus greggii-Dogleg Ash, Gregg Ash, Littleleaf Ash Fraxinus latifolia*-Basket Ash, Oregon Ash, Water Ash, White Ash Fraxinus nigra*-American Black Ash, Basket Ash, Black Ash , Brown Ash, Canadian Ash, Hoop Ash, Splinter Ash, Swamp Ash, Water Ash Fraxinus papillosa-Chihuahua Ash Fraxinus pennsylvanica*-Bastard Ash, Black Ash, Blue Ash, Brown Ash, Canadian Ash, Darlington Ash, Gray Ash, Green Ash , Piss Ash, Pumpkin Ash, Red Ash, Rim Ash, River Ash, Soft Ash,Swamp Ash, Water Ash, White Ash Fraxinus profunda*-Pumpkin Ash, Red Ash Fraxinus quadrangulata*-Blue Ash , Virginia Ash Fraxinus texensis-Texas Ash Fraxinus velutina-Arizona Ash, Desert Ash, Leatherleaf Ash, Modesto Ash, Smooth Ash, Toumey Ash, Velvet Ash (* commercial species) Distribution The north temperate regions of the globe. The Tree Ashes are trees or shrubs with large, opposite, pinnately compound leaves, which are shed in the fall. -

Are My Trees Ash?
F-55-04 School of Natural Resources, 2021 Coffey Road, Co lum bus, Ohio 43210 School of Natural Resources, 2021 Coffey Road, Columbus, Ohio 43210 Are My Trees Ash? Kathy L. Smith, Extension Associate, Forestry sh trees are common throughout Ohio’s rural and urban or toothed margins Alandscape. They are an important component of forests (Figure 8), and have throughout the state, with estimates suggesting that there may an oar-shaped fruit be as many as 3.8 billion ash trees growing naturally throughout called a samara (Figure Ohio’s forests. Ash trees are also one of our most frequently 3). White, green, and planted ornamental trees and are found planted in yards and black ash are prevalent along streets throughout most Ohio communities. across the state. Blue Four species of ash are relatively common in Ohio’s for- ash is more common ests — white ash (Fraxinus Americana), green ash (Fraxinus in the western part of pennsylvanica),), black ash (FraxinusFraxinus nigranigra),), and blue ash (FraxinusFraxinus the state. quadrangulata). Most of the numerous cultivars of ash planted Differentiating be- in the urban landscape are derived from these four species. A tween the four species fi fth species, pumpkin ash (Fraxinus profundaprofunda), is native to Ohio of ash that are com- but not commonly found in today’s forested landscape. White monly found in Ohio ash, green ash, black ash, blue ash, and pumpkin ash are all true isn’t easy. However, ashes; they are members of the genus Fraxinus. there are some key There are also a couple of other woody plants that have "ash" characteristics to look in their name, such as mountain ash, wafer ash, and prickly ash, for that will help you but these plants are not true ash. -

Native Vascular Flora of the City of Alexandria, Virginia
Native Vascular Flora City of Alexandria, Virginia Photo by Gary P. Fleming December 2015 Native Vascular Flora of the City of Alexandria, Virginia December 2015 By Roderick H. Simmons City of Alexandria Department of Recreation, Parks, and Cultural Activities, Natural Resources Division 2900-A Business Center Drive Alexandria, Virginia 22314 [email protected] Suggested citation: Simmons, R.H. 2015. Native vascular flora of the City of Alexandria, Virginia. City of Alexandria Department of Recreation, Parks, and Cultural Activities, Alexandria, Virginia. 104 pp. Table of Contents Abstract ............................................................................................................................................ 2 Introduction ...................................................................................................................................... 2 Climate ..................................................................................................................................... 2 Geology and Soils .................................................................................................................... 3 History of Botanical Studies in Alexandria .............................................................................. 5 Methods ............................................................................................................................................ 7 Results and Discussion .................................................................................................................... -

Middle Miocene Palynoflora of the Legnica Lignite Deposit Complex
Acta Palaeobotanica 49(1): 5–133, 2009 Middle Miocene palynofl ora of the Legnica lignite deposit complex, Lower Silesia, Poland ELŻBIETA WOROBIEC W. Szafer Institute of Botany, Polish Academy of Sciences, Lubicz 46, 31-512 Kraków, Poland; e-mail: [email protected] Received 18 November 2008; accepted for publication 11 May 2009 ABSTRACT. Palynological analysis of three profi les of the Miocene deposits from Legnica site (east fi eld, 33/56 and 41/52 profi les) and Ruja site (fragment of the Komorniki 97/72 profi le) has been presented. The samples consisted of material from the 2nd Lusatian lignite seam, the Mużaków series, the 1st Henryk lignite seam and grey clays of the Poznań series. The age of the fl ora was defi ned as Badenian (?Late Karpatian – Late Badenian). During the studies a total of 201 taxa from 96 genera (including 195 taxa from 92 genera of pollen and spores) were identifi ed. The systematic part of this work gives descriptions of selected sporomorphs and phytoplankton microfossils. Some informations about botanical affi nity, occurrence in fossil fl oras and in the studied material, as well as about allied recent plants are given in remarks. The results are presented in three pollen diagrams. The taxa have been classifi ed to an appropriate palaeofl oristical element mainly on the basis of the checklist of selected pollen and spores taxa from the Neogene deposits. The dominance of warm-temperate (A1) element and frequency of palaeotropical taxa in various parts of the profi les point to a warm-temperate climate. The results were used for reconstruction of changes in local vegetation during the sedimentation of deposits under study. -

8 March 2013, 381 P
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/273257107 Mason, P. G., D. R. Gillespie & C. Vincent (Eds.) 2013. Proceedings of the Fourth International Symposium on Biological Control of Arthropods. Pucón, Chile, 4-8 March 2013, 381 p. CONFERENCE PAPER · MARCH 2013 DOWNLOADS VIEWS 626 123 3 AUTHORS, INCLUDING: Peter Mason Charles Vincent Agriculture and Agri-Food Canada Agriculture and Agri-Food Canada 96 PUBLICATIONS 738 CITATIONS 239 PUBLICATIONS 1,902 CITATIONS SEE PROFILE SEE PROFILE Available from: Charles Vincent Retrieved on: 13 August 2015 The correct citation of this work is: Peter G. Mason, David R. Gillespie and Charles Vincent (Eds.). 2013. Proceedings of the 4th International Symposium on Biological Control of Arthropods. Pucón, Chile, 4-8 March 2013, 380 p. Proceedings of the 4th INTERNATIONAL SYMPOSIUM ON BIOLOGICAL CONTROL OF ARTHROPODS Pucón, Chile March 4-8, 2013 Peter G. Mason, David R. Gillespie and Charles Vincent (Eds.) 4th INTERNATIONAL SYMPOSIUM ON BIOLOGICAL CONTROL OF ARTHROPODS Pucón, Chile, March 4-8, 2013 PREFACE The Fourth International Symposium on Biological Control of Arthropods, held in Pucón – Chile, continues the series of international symposia on the biological control of arthropods organized every four years. The first meeting was in Hawaii – USA during January 2002, followed by the Davos - Switzerland meeting during September 2005, and the Christchurch – New Zealand meeting during February 2009. The goal of these symposia is to create a forum where biological control researchers and practitioners can meet and exchange information, to promote discussions of up to date issues affecting biological control, particularly pertaining to the use of parasitoids and predators as biological control agents. -

Wallander 2013
studiedagen – journées d’étude: fraxinus Systematics and fl oral evolution in Fraxinus (Oleaceae) Eva Wallander 1) Summary – Th e genus Fraxinus is one of 24 genera in the family Oleaceae. Fraxinus currently consists of 48 accepted tree and shrubby species distributed from the tropics to temperate regions of the northern hemisphere. About one third of the species is insect-pollinated and has small, white, scented fl owers borne many together in showy terminal panicles. Th e other two thirds are wind-pollinated, with apetalous and usually unisexual fl owers borne in tight lateral panicles or racemes. Unisexual fl owers have evolved on three separate occasions from bisexual ones in wind-pollinated species. Th e genus is divided into six sections: Fraxinus, Sciadanthus, Paucifl orae, Melioides, Ornus and Dipetalae. Th ese sections contain 45 species plus a few recognised subspecies. Th ree species are unclassifi ed due to uncertain positions in the phylogenetic tree. Th is latest classifi cation of the genus is based on an updated version of Wallander’s (2008) phylogenetic tree, which is based both on molecular and morphological data. A key to the sections is given as well as a systematic table with all accepted taxa, common syno- nyms and geographic distribution. Each section is presented along with some common, botanically interesting or commercially important species. Oleaceae, the olive family occur in Chionanthus and Fraxinus, and apetalous fl owers occur in Nestegis, Forestiera, Oleaceae is a family of about 600 species in 24 and wind-pollinated species of Fraxinus. Th e extant genera (Wallander & Albert 2000). ovary is syncarpous (carpels fused), consisting Th ey occur all over the world in tropical, sub- of two carpels. -

Njplantlist.Pdf
List of Endangered Plant Species and Plant Species of Concern June 2016 Scientific Name Common Name G Rank S Rank Federal Status State Status Other Status Abies balsamea Balsam Fir G5 S1 E LP, HL Acorus americanus American Sweetflag G5 S1? HL Actaea rubra var. rubra Red Baneberry G5T5 S2 HL Adlumia fungosa Climbing Fumitory G4 S2 HL Aeschynomene virginica Sensitive Joint-vetch G2 S1 LT E LP, HL Agalinis auriculata Ear-leaf False Foxglove G3 SX HL Agalinis fasciculata Pine Barren Foxglove G5 S3 HL Agalinis paupercula var. paupercula Small-flower False Foxglove G5T5 S2 HL Agastache nepetoides Yellow Giant-hyssop G5 S2 HL Agastache scrophulariifolia Purple Giant-hyssop G4 S2 HL Agrimonia microcarpa Small-fruit Grooveburr G5 S2 HL Agrostis geminata Ticklegrass G5 S1? HL Alisma triviale Large Water-plantain G5 S1 E LP, HL Alopecurus aequalis var. aequalis Short-awn Meadow-foxtail G5T5 S2 HL Alopecurus carolinianus Tufted Meadow-foxtail G5 S3 HL Amaranthus pumilus Seabeach Amaranth G2 S1 LT E LP, HL Amelanchier humilis Low Service-berry G5 S1S2 HL Amelanchier nantucketensis Nantucket Service-berry G3Q S1 HL Amelanchier sanguinea var. sanguinea Round-leaf Service-berry G5T5 S1.1 E LP, HL Amelanchier stolonifera Running Service-berry G5 S3 HL Amianthium muscitoxicum Fly Poison G4G5 S2 HL Ammannia latifolia Koehn's Toothcup G5 S1 E LP, HL Andromeda polifolia var. glaucophylla Bog Rosemary G5T5 S1 E LP, HL Andropogon glomeratus var. hirsutior Hairy Beardgrass G5T5 SH.1 HL Andropogon gyrans Elliott's Beardgrass G5 S2 HL Andropogon ternarius var. ternarius Silvery Beardgrass G5T5? S2 HL Anemone canadensis Canada Anemone G5 SX HL Anemone cylindrica Long-head Anemone G5 S1 E LP, HL Anemone virginiana var. -
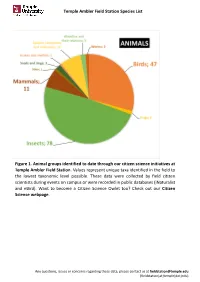
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

Fraxinus Biltmoreana and Fraxinus Smallii (Oleaceae), Forest Trees of the Eastern United States
Nesom, G.L. 2010. Fraxinus biltmoreana and Fraxinus smallii (Oleaceae), forest trees of the eastern United States. Phytoneuron 2010-51: 1–30. FRAXINUS BILTMOREANA AND FRAXINUS SMALLII (OLEACEAE), FOREST TREES OF THE EASTERN UNITED STATES GUY L. N ESOM 2925 Hartwood Drive Fort Worth, TX 76109, USA www.guynesom.com ABSTRACT Fraxinus biltmoreana Beadle and Fraxinus smallii Britton are recognized as species distinct from F. americana L., the white ash, closely following an earlier and informal delineation (Santamour 1962). Trees of F. americana sensu stricto are diploid, while those of F. smallii are tetraploid and those of F. biltmoreana are hexaploid. They differ by features of the fruits, leaf and twig vestiture, and shape and thickness of the petiole base; triploid and pentaploid hybrids apparently occur but are uncommon and probably sterile. Fraxinus americana (diploid) is more widely distributed to the west and north than the two polyploids, but the latter occur in 20 states of the eastern USA. Fraxinus smallii occurs from eastern Texas to Florida, north to Missouri, Ohio, and Pennsylvania; the range of F. biltmoreana is similar but it is rare in Arkansas, Louisiana, and Missouri and does not reach Texas. Differences in habitat appear to neglible or non-existent and two or all three of the species sometimes are encountered in close proximity. The distributions of F. americana , F. smallii , and F. biltmoreana are mapped at county level, and synonymy and typifications are provided for all three species. Other maps show the locations from which chromosome counts and estimates have been made. Fraxinus albicans Buckley, the Texas ash, and F. -

FDACS DPI Tri-Ology Volume 48, Number 1, March
DACS-P-00124 Volume 48, Number 3, May - June 2009 Printer-Friendly PDF Version DPI's Bureau of Entomology, Nematology and Plant Pathology (the botany section is included in this bureau) produces TRI-OLOGY six times a year, covering two months of activity in each issue. The report includes detection activities from nursery plant inspections, routine and emergency program surveys, and requests for identification of plants and pests from the public. Samples are also occasionally sent from other states or countries for identification or diagnosis. Highlights Following are a few of the notable entries from this volume of TRI-OLOGY. These entries Section Reports are reports of interesting plants or unusual pests, some of which may be problematic. See Section Reports for complete information. Botany Sacoila lanceolata (Aubl.) Garay. (beaked Entomology ladiestresses). This conspicuous and widespread Nematology species, also known as Spiranthes lanceolata and Stenorrhynchos lanceolatus, is one of Florida’s Plant Pathology showiest terrestrial orchids. It ranges from Florida and the West Indies, through Mexico and Central Our Mission…getting it done America, to South America east of the Andes as far south as Uruguay. In Florida, it is occasionally seen As a regulatory agency of the in flatwoods, oak hammocks, pastures, and roadsides Florida Department of throughout most of the peninsula, with an isolated Agriculture & Consumer occurrence in Walton County in the Panhandle. The Services, the Division of Plant species is included on the list of threatened plants by Industry works to detect, the State of Florida in order to draw attention to its intercept and control plant and vulnerability, although it is by no means rare at the honey bee pests that threaten Sacoila lanceolate Florida's native plant and moment. -

NYBG Field Guide to the Ash Trees of Northeastern United States
FIELD GUIDE TO THE ASH TREES OF NORTHEASTERN UNITED STATES 1 Field Guide to the Ash Trees of Northeastern United States CONTENTS Daniel Atha and Brian Boom Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Preface 3 Bronx, NY 10458 © 2017 The New York Botanical Garden Fraxinus 5 All rights reserved ISBN 978-0-89327-954-7 Key to the Identification ofFraxinus Species in Northeastern 5 United States and Adjacent Canada Citation Atha, D. and B. Boom. 2017. Field Guide to the Ash Trees of Northeastern United States. Center for Conservation Strategy, The New York Botanical Garden, Bronx, NY. 26 pp. Fraxinus americana (White Ash) 6 For additional copies of this publication, please contact the Center for Conservation Strategy, The New York Botanical Garden, 2900 Southern Boulevard, Bronx, NY Fraxinus biltmoreana (Biltmore Ash) 8 10458 U.S.A. Acknowledgments Fraxinus caroliniana (Water Ash) 10 The New York Botanical Garden gratefully acknowledges the Sarah K. de Coizart Article TENTH Perpetual Charitable Trust for its generous support of the project Strategy for Conserving Ash Trees in the Northeast: Collection, Analysis, and Outreach, Fraxinus excelsior (European Ash) 12 of which this field guide is one of the outcomes. Additional support was provided by the U.S. Department of Agriculture’s Agricultural Research Service (Agreement 58- 8020-6-004) for Characterization of Patterns of Genetic Diversity in North American Fraxinus nigra (Black Ash) 14 Ashes (Fraxinus spp.). The authors further thank their colleagues at The New York Botanical Garden for Fraxinus pennsylvanica (Green Ash, Red Ash) 16 valuable contributions to the project: Sandra Bruening, Sarah Hardy, Gregory Plunkett, Meryl Rubin, and Lisa Synoradzki.