View Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mmrv Vaccine
VACCINE INFORMATION STATEMENT (Measles, Mumps, Many Vaccine Information Statements are available in Spanish and other languages. MMRV Vaccine Rubella and See www.immunize.org/vis Varicella) Hojas de información sobre vacunas están disponibles en español y en muchos otros What You Need to Know idiomas. Visite www.immunize.org/vis These are recommended ages. But children can get the Measles, Mumps, Rubella and second dose up through 12 years as long as it is at least 1 Varicella 3 months after the first dose. Measles, Mumps, Rubella, and Varicella (chickenpox) can be serious diseases: Children may also get these vaccines as 2 separate shots: MMR (measles, mumps and rubella) and Measles varicella vaccines. • Causes rash, cough, runny nose, eye irritation, fever. • Can lead to ear infection, pneumonia, seizures, brain 1 Shot (MMRV) or 2 Shots (MMR & Varicella)? damage, and death. • Both options give the same protection. Mumps • One less shot with MMRV. • Causes fever, headache, swollen glands. • Children who got the first dose as MMRV have • Can lead to deafness, meningitis (infection of the brain had more fevers and fever-related seizures (about and spinal cord covering), infection of the pancreas, 1 in 1,250) than children who got the first dose as painful swelling of the testicles or ovaries, and, rarely, separate shots of MMR and varicella vaccines on death. the same day (about 1 in 2,500). Rubella (German Measles) Your doctor can give you more information, • Causes rash and mild fever; and can cause arthritis, including the Vaccine Information Statements for (mostly in women). MMR and Varicella vaccines. -

Vaccine Information for PARENTS and CAREGIVERS
NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES Division of the National Health Laboratory Service VACCINE INFOR MATION FOR PARENTS & CAREGIVERS First Edition November 2016 Editors-in-Chief Nkengafac Villyen Motaze (MD, MSc, PhD fellow), Melinda Suchard (MBBCh, FCPath (SA), MMed) Edited by: Cheryl Cohen, (MBBCh, FCPath (SA) Micro, DTM&H, MSc (Epi), Phd) Lee Baker, (Dip Pharm) Lucille Blumberg, (MBBCh, MMed (Micro) ID (SA) FFTM (RCPS, Glasgow) DTM&H DOH DCH) Published by: Ideas Wise and Wonderful (IWW) for National Institute for Communicable Diseases (NICD) First Edition: Copyright © 2016 Contributions by: Clement Adu-Gyamfi (BSc Hons, MSc), Jayendrie Thaver (BSc), Kerrigan McCarthy, (MBBCh, FCPath (SA), DTM&H, MPhil (Theol) Kirsten Redman (BSc Hons), Nishi Prabdial-Sing (PhD), Nonhlanhla Mbenenge (MBBCh, MMED) Philippa Hime (midwife), Vania Duxbury (BSc Hons), Wayne Howard (BSc Hons) Acknowledgments: Amayeza, Vaccine Information Centre (http://www.amayeza-info.co.za/) Centre for Communicable Diseases Fact Sheets (http://www.cdc.gov/vaccines/hcp/vis/) World Health Organization Fact Sheets (http://www.who.int/mediacentre/factsheets/en/) National Department of Health, South Africa (http://www.health.gov.za/) Disclaimer This book is intended as an educational tool only. Information may be subject to change as schedules or formulations are updated. Summarized and simplified information is presented in this booklet. For full prescribing information and contraindications for vaccinations, please consult individual package inserts. There has been -
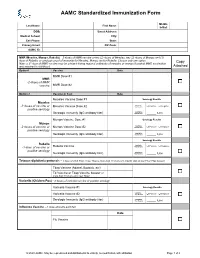
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

2021-22 School Year New York State Immunization Requirements for School Entrance/Attendance1
2021-22 School Year New York State Immunization Requirements for School Entrance/Attendance1 NOTES: Children in a prekindergarten setting should be age-appropriately immunized. The number of doses depends on the schedule recommended by the Advisory Committee on Immunization Practices (ACIP). Intervals between doses of vaccine should be in accordance with the ACIP-recommended immunization schedule for persons 0 through 18 years of age. Doses received before the minimum age or intervals are not valid and do not count toward the number of doses listed below. See footnotes for specific information foreach vaccine. Children who are enrolling in grade-less classes should meet the immunization requirements of the grades for which they are age equivalent. Dose requirements MUST be read with the footnotes of this schedule Prekindergarten Kindergarten and Grades Grades Grade Vaccines (Day Care, 1, 2, 3, 4 and 5 6, 7, 8, 9, 10 12 Head Start, and 11 Nursery or Pre-k) Diphtheria and Tetanus 5 doses toxoid-containing vaccine or 4 doses and Pertussis vaccine 4 doses if the 4th dose was received 3 doses (DTaP/DTP/Tdap/Td)2 at 4 years or older or 3 doses if 7 years or older and the series was started at 1 year or older Tetanus and Diphtheria toxoid-containing vaccine Not applicable 1 dose and Pertussis vaccine adolescent booster (Tdap)3 Polio vaccine (IPV/OPV)4 4 doses 3 doses or 3 doses if the 3rd dose was received at 4 years or older Measles, Mumps and 1 dose 2 doses Rubella vaccine (MMR)5 Hepatitis B vaccine6 3 doses 3 doses or 2 doses of adult hepatitis B vaccine (Recombivax) for children who received the doses at least 4 months apart between the ages of 11 through 15 years Varicella (Chickenpox) 1 dose 2 doses vaccine7 Meningococcal conjugate Grades 2 doses vaccine (MenACWY)8 7, 8, 9, 10 or 1 dose Not applicable and 11: if the dose 1 dose was received at 16 years or older Haemophilus influenzae type b conjugate vaccine 1 to 4 doses Not applicable (Hib)9 Pneumococcal Conjugate 1 to 4 doses Not applicable vaccine (PCV)10 Department of Health 1. -

Vaccinations for Adults with Chronic Liver Disease Or Infection
Vaccinations for Adults with Chronic Liver Disease or Infection This table shows which vaccinations you should have to protect your health if you have chronic hepatitis B or C infection or chronic liver disease (e.g., cirrhosis). Make sure you and your healthcare provider keep your vaccinations up to date. Vaccine Do you need it? Hepatitis A Yes! Your chronic liver disease or infection puts you at risk for serious complications if you get infected with the (HepA) hepatitis A virus. If you’ve never been vaccinated against hepatitis A, you need 2 doses of this vaccine, usually spaced 6–18 months apart. Hepatitis B Yes! If you already have chronic hepatitis B infection, you won’t need hepatitis B vaccine. However, if you have (HepB) hepatitis C or other causes of chronic liver disease, you do need hepatitis B vaccine. The vaccine is given in 2 or 3 doses, depending on the brand. Ask your healthcare provider if you need screening blood tests for hepatitis B. Hib (Haemophilus Maybe. Some adults with certain high-risk conditions, for example, lack of a functioning spleen, need vaccination influenzae type b) with Hib. Talk to your healthcare provider to find out if you need this vaccine. Human Yes! You should get this vaccine if you are age 26 years or younger. Adults age 27 through 45 may also be vacci- papillomavirus nated against HPV after a discussion with their healthcare provider. The vaccine is usually given in 3 doses over a (HPV) 6-month period. Influenza Yes! You need a dose every fall (or winter) for your protection and for the protection of others around you. -

Vaccinations for Preteens and Teens, Age 11-19 Years
Vaccinations for Preteens and Teens, Age 11–19 Years Getting immunized is a lifelong, life-protecting job. Make sure you and your healthcare provider keep your immunizations up to date. Check to be sure you’ve had all the vaccinations you need. Vaccine Do you need it? Chickenpox Yes! If you haven’t been vaccinated and haven’t had chickenpox, you need 2 doses of this vaccine. (varicella; Var) Anybody who was vaccinated with only 1 dose should get a second dose. Hepatitis A Yes! If you haven’t been vaccinated, you need 2 doses of this vaccine. Anybody who was vaccinated (HepA) with only 1 dose should get a second dose at least 6–18 months later. Hepatitis B Yes! This vaccine is recommended for all people age 0–18 years. You need a hepatitis B vaccine series (HepB) if you have not already received it. Haemophilus influ- Maybe. If you haven’t been vaccinated against Hib and have a high-risk condition (such as a non- enzae type b (Hib) functioning spleen), you need this vaccine. Human Yes! All preteens need 2 doses of HPV vaccine at age 11 or 12. Older teens who haven't been vacci- papillomavirus nated will need 2 or 3 doses. This vaccine protects against HPV, a common cause of genital warts and (HPV) several types of cancer, including cervical cancer and cancer of the anus, penis, and throat. Influenza Yes! Everyone age 6 months and older needs annual influenza vaccination every fall or winter and (Flu) for the rest of their lives. -

Adult Immunizations
Guidelines for Clinical Care Ambulatory Immunizations Guideline Team Adult Immunizations Team Leads Susan F Engert, MD, MPH Population: Adults, >18 years old Pediatrics and Communi- cable Diseases Objectives: Implement an evidence-based strategy for routine adult immunizations. Candia B Laughlin, RN, Key Points MS Routine immunizations for adults are: hepatitis A, hepatitis B, herpes zoster, human papilloma virus, Ambulatory Care Nursing Administration influenza, measles, mumps, rubella, meningococcal, pneumococcal, tetanus, diphtheria, pertussis and Team Members varicella. Below is a summary on priority populations, initial vaccination, and revaccination. Margie C Andreae, MD Use combination vaccines whenever possible to increase the coverage rates for vaccine-preventable Pediatrics and Communi- diseases: Tetanus-diphtheria (Td), Tetanus-diphtheria-acellular pertussis (Tdap), Measles-Mumps- cable Diseases Rubella (MMR), hepatitis A-hepatitis B (Twinrix®). Single antigen vaccines have no safety advantage. Mary K.Barry-Bodine, Live virus vaccines (Herpes Zoster, Measles-Mumps-Rubella, Varicella and Live Attenuated Influenza RN, BSN Vaccine) are contraindicated in persons who are pregnant or may become pregnant in the next four Nursing, Health Centers weeks, or who have immunocompromising conditions. If administering multiple live vaccines, give Susan G Blitz, MD, MPH simultaneously or separate them by 4 weeks. Tuberculosis (PPD) skin test should be administered General Medicine before or on the same day as a live virus vaccine or they need to be spaced 4-6 weeks apart. Katie Barwig, RN, MS This guideline follows recommendations of the federal Advisory Committee on Immunization Practices: Nursing Administration These vaccinations should be performed [strength of recommendation] for indicated populations at risk. Sherry L DeLoach, Evidence for each vaccine is based on randomized controlled trials [level of evidence] in general PharmD population and some subgroups, with findings extrapolated to some subgroups. -

Healthcare Worker Vaccination Recommendations
Healthcare Personnel Vaccination Recommendations1 Vaccine Recommendations in brief Hepatitis B Give 3-dose series (dose #1 now, #2 in 1 month, #3 approximately 5 months after #2). Give IM. Obtain anti-HBs serologic testing 1–2 months after dose #3. Influenza Give 1 dose of influenza vaccine annually. Give inactivated injectable influenza vaccine intramuscularly or live attenuated influenza vaccine (LAIV) intranasally. MMR For healthcare personnel (HCP) born in 1957 or later without serologic evidence of immunity or prior vaccination, give 2 doses of MMR, 4 weeks apart. For HCP born prior to 1957, see below. Give SC. Varicella For HCP who have no serologic proof of immunity, prior vaccination, or history of varicella disease, (chickenpox) give 2 doses of varicella vaccine, 4 weeks apart. Give SC. Tetanus, diphtheria, Give a one-time dose of Tdap as soon as feasible to all HCP who have not received Tdap previously. pertussis Give Td boosters every 10 years thereafter. Give IM. Meningococcal Give 1 dose to microbiologists who are routinely exposed to isolates of N. meningitidis. Give IM or SC. Hepatitis A, typhoid, and polio vaccines are not routinely recommended for HCP who may have on-the-job exposure to fecal material. Hepatitis B level of immunity upon testing should be considered nonimmune) or (b) Healthcare personnel (HCP) who perform tasks that may involve exposure to appropriate vaccination against measles, mumps, and rubella (i.e., 2 doses blood or body fluids should receive a 3-dose series of hepatitis B vaccine at of live measles and mumps vaccines given on or after the first birthday, 0-, 1-, and 6-month intervals. -

CDC-Measles-Provider-Letter.Pdf
Dear Provider, Due to the current increase in measles cases in the United States (https://www.cdc.gov/mmwr/volumes/68/wr/mm6817e1.htm), the Centers for Disease Control and Prevention has developed the following summary for vaccination of adults against measles with measles, mumps, rubella (MMR) vaccine. Recommendations for vaccination and assessing immunity in adults have not changed since publication of the Advisory Committee on Immunization Practices (ACIP) recommendations for the Prevention of Measles, Rubella, Congenital Rubella syndrome, and Mumps in June 2013. (https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm) WHAT ADULT PROVIDERS NEED TO KNOW: • Providers do not need to actively screen adult patients for measles immunity. This is because of high population immunity and low risk of disease among adults in non-outbreak areas in the U.S. Providers should make sure patients have measles protection before international travel. U.S. residents traveling internationally are at high risk for acquiring measles abroad. They can also transmit measles to susceptible persons, such as infants, when they return home. • If a patient is traveling internationally and measles immunity is unknown, providers should vaccinate, unless there are contraindications. Serologic testing for measles immunity is not recommended. • During outbreaks, providers should consult with local health departments for the most up-to-date recommendations for their community. This may include additional doses of MMR for your patients. Measles is an acute viral illness characterized by a prodrome of fever, cough, coryza, and conjunctivitis, followed by a maculopapular rash. The rash spreads from the head to trunk to the lower extremities. -

Recommendations for Immunizations and TB Testing for Health Science Students
Recommendations for Immunizations and TB Testing for Health Science Students Overview Influenza: 1 dose of inactivated Influenza vaccine yearly. Hepatitis B: 3-dose series of hepatitis B vaccine given at 0, 1 and 6 months AND documented quantitative hepatitis B surface antibody titer consistent with immunity after the appropriate vaccine series. Measles/Mumps/Rubella (MMR): 2 doses of MMR vaccine at least 28 days apart after 12 months of age OR 2 doses of measles and 2 doses of Mumps at least 28 days apart after 12 months of age and one dose of rubella after 12 months of age OR laboratory proof of immunity to measles/mumps/rubella. Tetanus/Diphtheria/Pertussis: In addition to primary series, all Health Care Personnel (HCP) should receive 1 dose of Tdap and have documentation of a Td or Tdap within the past 10 years. Tuberculosis Testing: The CDC recommends initial base line testing with a 2-step TB skin test or a blood test for TB infection. Subsequent annual or serial screening is determined by state regulations or risk assessment. Varicella: 2 doses of varicella vaccine given at least 4 weeks apart OR laboratory proof of immunity for those with a history of disease. If titer is negative or equivocal, give 2-dose varicella vaccine series. Do not repeat titer after series completion. Hepatitis B: Students must have a series of 3 hepatitis B vaccines AND a positive (≥10 mIU/mL) serological quantitative Hepatitis B surface antibody titer (anti-HBs or HBsAb) that was performed at least 1-2 months after the 3rd dose of hepatitis B vaccine. -

IDSA Releases Recommendations on Vaccinations in Immunocompromised Patients
Practice Guidelines IDSA Releases Recommendations on Vaccinations in Immunocompromised Patients ciency; those with secondary immunodefi- Key Points for Practice ciency caused by human immunodeficiency • Vaccines should be administered before planned immunosuppression, virus (HIV) infection, cancer chemother- with live vaccines given four weeks in advance and inactivated vaccines given two weeks in advance. apy, stem cell or solid organ transplant, • Immunocompetent persons who live with an immunocompromised sickle cell disease, and surgical asplenia; patient can safely receive inactivated vaccines. and patients with chronic inflammatory • Varicella and zoster vaccines should not be administered to highly diseases who are receiving systemic corti- immunocompromised patients. costeroids, immunomodulators, or biologic • Annual vaccination with inactivated influenza vaccine is recommended agents. The guideline includes several tables, for immunocompromised patients six months and older, except those one for each condition, that list specific who are unlikely to respond. vaccines that are recommended and contra- From the AFP Editors indicated, with the level of evidence associ- ated with each recommendation. Some of Coverage of guidelines Vaccination of immunocompromised the recommendations distinguish between from other organizations patients is important because impaired host high- and low-level immunosuppression. does not imply endorse- ment by AFP or the AAFP. defenses predispose patients to an increased High-level immunosuppression includes risk of vaccine-preventable infections. These patients who have a primary immunode- A collection of Practice Guidelines published in patients also have a greater risk of exposure ficiency; who are receiving chemotherapy; AFP is available at http:// to pathogens because of their frequent con- who have received a solid organ transplant www.aafp.org/afp/ tact with medical environments. -

MMR Vaccine Information
MEASLES MUMPS & RUBELLAVACCINES ( W H A T Y O U N E E D T O K N O W ) _________ These are the recommended ages. But children can c1 I Why get vaccinated? ) get the second dose at any age, as long as it is at least 28 days after the first dose. Measles, mumps, and rubella are serious diseases. Some adults should also get MMR vaccine: Measles Generally, anyone 18 years of age or older, who was • Measles virus causes rash, cough, runny nose, eye born after 1956, should get at least one dose of irritation, and fever. MMR vaccine, unless they can show that they have • It can lead to ear infection, pneumonia, seizures had either the vaccines or the diseases. (jerking and staring), brain damage, and death. Ask your doctor or nurse for more information. Mumps • Mumps virus causes fever, headache, and swollen MMR vaccine may be given at the same time as glands. other vaccines. • It can lead to deafness, meningitis (infection of the brain and spinal cord covering), painful swelling of the testicles or ovaries, and, rarely, death. Some people should not get C_I3 MMR ___ vaccine or should wait ) Rubella (German Measles) • Rubella virus causes rash, mild fever, and arthritis (mostly in women). • People should not get MMR vaccine who have • If a woman gets rubella while she is pregnant, she ever had a life-threatening allergic reaction to could have a miscarriage or her baby could be gelatin, the antibiotic neomycin, or to a previous born with serious birth defects. dose of MMR vaccine.