Grades of Nylon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
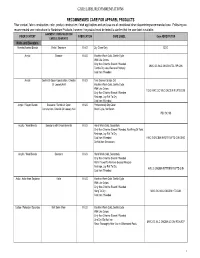
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

Choosing the Proper Short Cut Fiber for Your Nonwoven Web
Choosing The Proper Short Cut Fiber for Your Nonwoven Web ABSTRACT You have decided that your web needs a synthetic fiber. There are three important factors that have to be considered: generic type, diameter, and length. In order to make the right choice, it is important to know the chemical and physical characteristics of the numerous man-made fibers, and to understand what is meant by terms such as denier and denier per filament (dpf). PROPERTIES Denier Denier is a property that varies depending on the fiber type. It is defined as the weight in grams of 9,000 meters of fiber. The current standard of denier is 0.05 grams per 450 meters. Yarn is usually made up of numerous filaments. The denier of the yarn divided by its number of filaments is the denier per filament (dpf). Thus, denier per filament is a method of expressing the diameter of a fiber. Obviously, the smaller the denier per filament, the more filaments there are in the yarn. If a fairly closed, tight web is desired, then lower dpf fibers (1.5 or 3.0) are preferred. On the other hand, if high porosity is desired in the web, a larger dpf fiber - perhaps 6.0 or 12.0 - should be chosen. Here are the formulas for converting denier into microns, mils, or decitex: Diameter in microns = 11.89 x (denier / density in grams per milliliter)½ Diameter in mils = diameter in microns x .03937 Decitex = denier x 1.1 The following chart may be helpful. Our stock fibers are listed along with their density and the diameter in denier, micron, mils, and decitex for each: Diameter Generic Type -

Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing
machines Article Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing Flaviana Calignano 1,* , Massimo Lorusso 2 , Ignanio Roppolo 3 and Paolo Minetola 1 1 Department of Management and Production Engineering, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] 2 Istituto Italiano di Tecnologia, Center for Sustainable Future Technologies IIT@Polito, Corso Trento 21, 10129 Turin, Italy; [email protected] 3 Department of Applied Science and Technology, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] * Correspondence: fl[email protected]; Tel.: +39-011-090-7218 Received: 19 July 2020; Accepted: 2 September 2020; Published: 4 September 2020 Abstract: Additive manufacturing (i.e., 3D printing) has rapidly developed in recent years. In the recent past, many researchers have highlighted the development of in-house filaments for fused filament fabrication (FFF), which can extend the corresponding field of application. Due to the limited mechanical properties and deficient functionality of printed polymer parts, there is a need to develop printable polymer composites that exhibit high performance. This study analyses the actual mechanical characteristics of parts fabricated with a low-cost printer from a carbon fibre-reinforced nylon filament. The results show that the obtained values differ considerably from the values presented in the datasheets of various filament suppliers. Moreover, the hardness and tensile strength are influenced by the building direction, the infill percentage, and the thermal stresses, whereas the resilience is affected only by the building direction. Furthermore, the relationship between the mechanical properties and the filling factor is not linear. -

The Pennsylvania State University the Graduate School AN
The Pennsylvania State University The Graduate School AN EXAMINATION OF ANALYTICAL METHODS TOWARDS THE COMPLETE ANALYSIS OF CONTAMINANTS OF EMERGING CONCERN IN WASTEWATER AND WASTEWATER IMPACTED SURFACE WATER, SOILS, AND CROPS. A Dissertation in Chemistry by Kyra A. Murrell © 2020 Kyra A. Murrell Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2020 The dissertation of Kyra A. Murrell was reviewed and approved by the following: Frank L. Dorman Associate Professor of Biochemistry and Molecular Biology Dissertation Co-Advisor Co-Chair of Committee Miriam Freedman Associate Professor of Chemistry, Meteorology and Atmospheric Science Dissertation Co-Adviser Co-Chair of Committee Paul Cremer J. Lloyd Huck Professor of Chemistry and of Biochemistry and Molecular Biology Christine Keating Distinguished Professor of Chemistry Jack Watson Professor of Soil Science, Soil Physics, Biogeochemistry Philip Bevilacqua Distinguished Professor of Chemistry and Biochemistry and Molecular Biology Department Head, Chemistry iii ABSTRACT The presence of contaminants of emerging concern (CECs) in the environment is a growing field of research for analytical environmental scientists. CECs are a class of anthropogenic pollutants not regulated by governmental agencies, and their potential deleterious environmental and human impacts are largely unknown. One of the main sources of CEC entry into the aquatic environment is wastewater treatment plant (WWTP) effluent as the treated water is often released into bodies of water, such as river and streams. Because most WWTPs were not designed to remove organic micropollutants, many CECs are poorly removed in traditional WWTPs and persist in the treated effluent waters. As a model system for study, the University Park WWTP treats the wastewater from the Penn State main campus. -

Nylon Wool Fiber Columns
U.S. Corporate Headquarters Polysciences Europe GmbH Polysciences Asia-Pacific, Inc. 400 Valley Rd. Badener Str. 13 2F-1, 207 DunHua N. Rd. Warrington, PA 18976 69493 Hirschberg an der Taipei, Taiwan 10595 1(800) 523-2575 / (215) 343-6484 Bergstrasse, Germany (886) 2 8712 0600 1(800)343-3291 fax +(49) 6201 845 20 0 (886) 2 8712 2677 fax [email protected] +(49) 6201 845 20 20 fax [email protected] [email protected] TECHNICAL DATA SHEET 425A Page 1 of 2 Nylon Wool Fiber Columns BACKGROUND NYLON WOOL FIBER VS. SHEEP RBC ROSETTING METHODS Researchers have been using nylon wool fiber procedures to separate T-cell and Wong and Mittal (1981)9 did extensive research comparing the methods of Nylon B-cell lymphocytes for more than 20 years. In the early 1970’s M. H. Julius et al Wool Fiber separation and the commonly- used and well-studied sheep RBC (1973),1 Eisen et al (1972),2 and Greaves & Brain (1974)3 described specific (SRBC) rosetting.10,11 Wong and Mittal were interested in isolating B-cells for conditions for the use of Nylon Wool Fiber in columns or plastic straws. These serologic typing of HLA-DR antigen. protocols resulted in yields of 50-90% T-cell recovery and 10-100 fold B-cell depletion. Wong and Mittal concluded that “Due to its simplicity and reliability, nylon wool adherence may be preferred over the SRBC rosette method for the routine pheno- These early researchers found it necessary to scrub or wash their Nylon Wool Fiber typing of B-cells.” Their findings are illustrated in Table 1. -

PEP Review 2015-06 Polyamide (Nylon) 6 and 66 Process Summary
IHS CHEMICAL PEP Review 2015-06 Polyamide (Nylon) 6 and 66 Process Summary July 2015 ihs.com PEP Review Process Economics Program Dipti Dave Senior Analyst II IHS CHEMICAL | Process Economics Program Review 2015-06 PEP Review 2015-06 Polyamide (Nylon) 6 and 66 Process Summary Dipti Dave, Senior Analyst II Abstra ct Polyamide 6 and 66 (or Nylon 6 and 66) are the most common types of polyamide available commercially. The total volume for the Nylon 6 and 66 polymerization market is 7.2 million tons in 2014, up from 6.4 million tons in 2010. Nylon 6 and 66 polymerization produces either chips or resin in uniform pellets. The chips or resin are further processed into two major applications: fibers or engineering thermoplastics (ETP). The fibers may also be directly produced from the molten state of the polymer, bypassing chip/resin production. The majority of the Nylon chip or resin production accounts for 92% of total polymerization, while fiber production (directly from melting) accounts for 8% market share. Demand is expected to grow at an average annual growth rate (AAGR) of 2.4% for Nylon 6 ETP and fiber. The AAGR for Nylon 66 ETP and fiber demand is 2.6%. Capacity additions have been taking place mostly in China. The Nylon processes have been reviewed by IHS Chemical Process Economics Program (PEP) since its inception in 1962. In this process summary, we review the key features for Nylon 6 and 66 production processes, and discuss recent technology developments and update the process economics for the following Nylon 6 and 66 stand-alone and integrated processes presented: 1. -

Interfacial Adhesion in Rayon/Nylon Sheath/Core Composite Fibers. Weiying Tao Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1991 Interfacial Adhesion in Rayon/Nylon Sheath/Core Composite Fibers. Weiying Tao Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Tao, Weiying, "Interfacial Adhesion in Rayon/Nylon Sheath/Core Composite Fibers." (1991). LSU Historical Dissertations and Theses. 5213. https://digitalcommons.lsu.edu/gradschool_disstheses/5213 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Immersion Dyeing Nylon and Acetate Rayon Using Prosperse Disperse Dyes Please Read Directions Carefully Before Starting
Immersion Dyeing Nylon and Acetate Rayon using PROsperse Disperse Dyes Please read directions carefully before starting. For medium to dark shades, it is recommended that nylon be dyed with acid dyes, because disperse dyes lack acceptable fastness. Acetate rayon can only be dyed with disperse dyes and has acceptable fastness in all depths of shade with the disperse dyes. All Dyeing should be done in a stainless steel or enamelware container only. Never use aluminum pots. Use Pyrex or stainless steel measuring utensils and a large wooden dowel for stirring in the boiling dye bath. Always do test samples before working on a large project. Please Note: These dyes have the potential to stain any sink that is not made of stainless steel or fireclay ceramic. For additional information, visit our web site at www.prochemicalanddye.com. Wear rubber gloves, apron, or old clothes. Utensils used for dyeing should never be used for food preparation. Supplies PROsperse Disperse Dye Citric Acid Crystals or White Distilled Vinegar Synthrapol PRO Dye Activator or Soda Ash Procedure 1. Scour the fabric by machine washing in HOT 140F (60C) water, or by hand in a pot on the stove with 2 tsp (2 gm) PRO Dye Activator or Soda Ash and 2 tsp (2.5 ml) Synthrapol per pound of fabric (454 gm, or 3 to 4 yards of muslin weight fabric). Rinse thoroughly. This step does not add the dye fixative to the fabric; it prepares your fabric for dyeing by removing any dirt, oil or sizing. 2. Dissolve the dye. Thoroughly dissolve the desired amount of dye powder, from the chart below, in 1 cup (250 ml) of boiling water. -

Synthesis and Characterization of In-Situ Nylon-6/Epoxy Blends
Synthesis and Characterization of in-situ Nylon-6/Epoxy Blends A thesis submitted to the Division of Research and Advanced Studies University of Cincinnati In partial fulfillment of the requirements for the degree of Master of Science 2016 In the Materials Science and Engineering Program, The Department of Mechanical and Materials Engineering By Anushree Deshpande B.E Polymer, University of Pune, 2011 Committee Members: Dr. Jude O. Iroh (Chair) Dr. Relva C. Buchanan Dr. Raj M. Manglik 1 ABSTRACT Epoxy is a thermosetting polymer known for its excellent adhesion, thermal stability, chemical resistance and mechanical properties. However, one of the major drawbacks of epoxies is its inherent brittleness. In order to overcome this drawback, incorporation of a thermoplastic as a second phase has proven to improve the impact strength without affecting the mechanical properties of epoxy. Researchers in the past have studied polyamide/epoxy blends in terms of blend compatibility, thermo-mechanical properties and morphology via solution blending. The current research effort employs in-situ polymerization to synthesize polyamide/epoxy blends. Blends of various compositions were synthesized by introducing Ɛ-Caprolactam (monomer of nylon-6) in the prepolymer of epoxy. All blend fractions were cured by exposing them to the same time and temperature conditions; and characterized using Dynamic Mechanical Analysis (DMA), Fourier Transform Infrared Spectroscopy (FTIR), Brookfield Viscometry, Scanning Electron Microscopy (SEM) and Thermogravimetric Analysis (TGA). DMA results show an overall increase in glass transition temperature and storage modulus in the rubbery region. FTIR results reveal maximum epoxy curing up to 15 wt% monomer loading, beyond which the plateauing of the epoxy conversion is recognized. -

Carpet Recycling
1 TheThe ParticipantParticipant willwill gaingain knowledgeknowledge inin thethe following:following: › Drivers for Carpet Recycling › General Categories of carpet recycling › Differences in Various types of recycling › Market Values of Various Recycled Products › Demand for Recycled materials from Carpet › Understanding Capital needs of Recycling › Present & Anticipated recycling capacities › Present & New Recycling technologies › Challenges & Opportunities 2 3 BroadBroad ListList ofof Drivers:Drivers: › Carpet Manufacturers › LEED building Standards –Need P. Consumer for high value/Specifications › NSF 140 › High value of P.C. content › Platinum Level highly prized: Requires Min. Post consumer content. › Platinum Level requires P.C. Carpet recycling at CARE Goal levels – Escalate every year. › Professional Specifying Commercial Community demands Sustainability › Reward most Sustainable companies with increased business: or NO business › Recycling and P. Consumer recycled content is large factor › Large National Accounts demanding sustainable initiatives: › Wal‐Mart, Home Depot, etc. › Good Old healthy competition. 4 BroadBroad ListList ofof Drivers:Drivers: › Entrepreneurs: › Willing to risk Capital for carpet recycling › They are beginning to see fairly good business model › Beginning to make money from carpet recycling › They are essential link in the value chain of processing › High Oil prices › Keeps Virgin Nylon very expensive › Cost Spread between virgin and P. Consumer is wide › Makes P. Consumer very attractive for cost savings -

Colaris Digital Textile Printing
ZIMMER AUSTRIA | DIGITAL PRINTING SYSTEMS COLARIS DIGITAL TEXTILE PRINTING HOME TEXTILES APPAREL DECORATION AUTOMOTIVE FLAGS & BANNERS www.zimmer-austria.com 2020.01.15 page 1 CONTENT 1. INNOVATION IS IN OUR DNA 1.1. HISTORIC MILESTONES 3 2. INK CLASSES 2.1. TYPES | PRODUCTS | PROCESS | REQUIREMENTS 4 2.2. TYPES | PRODUCTS | PROCESS | REQUIREMENTS 5 3. PRINT TECHNOLOGY 3.1. PROCESSING DIAGRAM 6 3.2. PROCESS EQUIPMENT 7 4. REACTIVE PRINTING 4.1. GENERAL INFORMATION 8 4.2. EXAMPLE: TERRY TOWEL PRINT PRODUCTION 9 5. ACID PRINTING 5.1. GENERAL INFORMATION 10 5.2. EXAMPLE: UPHOLSTERY PRINT LINE 11 6. DISPERSE / SUBLIMATION PRINTING 6.1. GENERAL INFORMATION 12 6.2. EXAMPLE: PES BLANKET PRINT LINE 13 7. VAT INDANTHRENE® PRINTING 7.1. GENERAL INFORMATION 14 7.2. APPLICATION DIVERSITY 15 8. PIGMENT PRINTING 8.1. GENERAL INFORMATION 16 8.2. APPLICATION DIVERSITY 17 9. CATIONIC PRINTING 9.1. GENERAL INFORMATION 18 10. COLARIS - CHARACTERISTICS AND FEATURES 10.1. COLARIS MODELS 19 11. COLARIS FEATURES AND COMPONENTS 11.1. INTEGRATED MACHINE COMPONENTS 20 11.2. INTEGRATED MACHINE COMPONENTS 21 12. PROCESS EQUIPMENT 12.1. INLINE COMPONENTS 22 12.2. OFFLINE COMPONENTS 23 13. PRINT HEAD 13.1. TECHNOLOGY 24 13.2. RECONDITION CENTER 25 14. ZIMMER TECHNOLOGY & APPLICATION CENTER 14.1. GENERAL INFORMATION 26 14.2. EQUIPMENT & FACILITIES 27 www.zimmer-austria.com 2020.01.15 page 2 1. INNOVATION IS IN OUR DNA 1.1. HISTORIC MILESTONES Vertical Duplex blanket printer from 1951 First commercial rotary screen printer 1958 The broad digital competence of ZIMMER AUSTRIA is based on an innovation introduced more than 4 decades ago.