Agonists: Fensolvi (Leuprolide), Lupron Depot 3.75
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparison of the Costs for Treating Central Precocious Puberty During the First Year with Monthly Leuprolide Acetate Injectable and Histrelin Acetate Implant
A Comparison Of The Costs For Treating Central Precocious Puberty During The First Year With Monthly Leuprolide Acetate Injectable And Histrelin Acetate Implant B F Banahan III1, Mayo K2, Summers KH2 1 Center for Pharmaceutical Marketing and Management and Department of Pharmacy Administration, University of Mississippi, University, MS 2 Health Outcomes & PharmacoEconomics, Endo Pharmaceuticals, Inc., Chadds Ford, PA Estimated Annual Cost of Treatment if all PTs Treated With . BACKGROUND METHODS MEDICAID MODEL Lupron SUPPRELIN LA Product costs $ 1,770,201 $ 1,515,188 Two retrospective cohort studies were conducted using datasets derived from the Office visit costs $ 79,896 $ 14,000 Puberty results when secretion of gonadotropin releasing hormone (GnRH) is initiated 100% compliance with Lupron treatment 1 Implant procedures $ 91,400 and the hypothalamic-pituitary-gonadal axis is activated. During puberty, the brain Thomas Reuter’s MarketScan© Multi-State Medicaid Database (2003-2007) and the (All patients receive 13+ treatments in year) MarketScan© Commercial Database (2005-2009). A probabilistic patient flow model Lab/xray for monitoring $ 53,900 $ 37,500 produces GnRH through a complex process. GnRH causes increases in other TOTAL COST TO PAYER $ 1,903,997 $ 1,658,088 hormones like luteinizing hormone (LH) and follicle stimulating hormone (FSH). It is was developed using estimates for treatment patterns and costs for products, office Normal Compliance with Lupron: Lupron SUPPRELIN LA these hormones that cause the ovaries to produce estrogen and the testicles to visits, and monitoring therapy. Patients with < 13 treatments per year Product costs $ 1,572,921 $ 1,515,188 Quality of Care ofCare Quality % of TXd PTs: 53% Aver. -

Hormones and Breeding
IN-DEPTH: REPRODUCTIVE ENDOCRINOLOGY Hormones and Breeding Carlos R.F. Pinto, MedVet, PhD, Diplomate ACT Author’s address: Theriogenology and Reproductive Medicine, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, The Ohio State University, Columbus, OH 43210; e-mail: [email protected]. © 2013 AAEP. 1. Introduction affected by PGF treatment to induce estrus. In The administration of hormones to mares during other words, once luteolysis takes place, whether breeding management is an essential tool for equine induced by PGF treatment or occurring naturally, practitioners. Proper and timely administration of the events that follow (estrus behavior, ovulation specific hormones to broodmares may be targeted to and fertility) are essentially similar or minimally prevent reproductive disorders, to serve as an aid to affected (eg, decreased signs of behavioral estrus). treating reproductive disorders or hormonal imbal- Duration of diestrus and interovulatory intervals ances, and to optimize reproductive efficiency, for are shortened after PGF administration.1 The example, through induction of estrus or ovulation. equine corpus luteum (CL) is responsive to PGF These hormones, when administered exogenously, luteolytic effects any day after ovulation; however, act to control the duration and onset of the different only CL Ͼ5 days are responsive to one bolus injec- stages of the estrous cycle, specifically by affecting tion of PGF.2,3 Luteolysis or antiluteogenesis can duration of luteal function, hastening ovulation es- be reliably achieved in CL Ͻ5 days only if multiple pecially for timed artificial insemination and stimu- PGF treatments are administered. For that rea- lating myometrial activity in mares susceptible to or son, it became a widespread practice to administer showing delayed uterine clearance. -

DESCRIPTION Vantas™ (Histrelin Implant) Is a Sterile Non-Biodegradable, Diffusion-Controlled Reservoir Drug Delivery System De
Vantas™ (histrelin implant) PACKAGE INSERT DESCRIPTION Vantas™ (histrelin implant) is a sterile non-biodegradable, diffusion-controlled reservoir drug delivery system designed to deliver histrelin continuously for 12 months upon subcutaneous implantation. The Vantas implant contains 50 mg of histrelin acetate. Histrelin acetate is a synthetic nonapeptide analogue of the naturally occurring gonadotropin releasing hormone (GnRH) or luteinizing hormone releasing hormone (LH-RH). The sterile Vantas implantation device (provided with the implant) is used to insert the implant subcutaneously in the inner aspect of the upper arm. After 12 months, the implant must be removed. At the time the implant is removed, another implant may be inserted to continue therapy. The sterile Vantas ™ implant consists of a 50-mg histrelin acetate drug core inside a non- biodegradable, 3 cm by 3.5 mm cylindrically shaped hydrogel reservoir (Figure A). The drug core also contains the inactive ingredient stearic acid NF. The hydrogel reservoir is a hydrophilic polymer cartridge composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100. The hydrated implant is packaged in a glass vial containing 2.0 mL of 1.8% NaCl solution. The implant is primed for release of the drug upon insertion. Figure A. Vantas Histrelin Implant diagram (not to scale) Hydrogel Reservoir Drug Formulation Histrelin acetate is chemically described as 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L- tyrosyl-Nt-benzyl-D-histidyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) [C66H86N18O12. (1.7-2.8 moles) CH3COOH, (0.6-7.0 moles) H2O], with the molecular weight of 1443.70 (or 1323.50 as histrelin base. -

PE2572 Puberty Blockers
Puberty Blockers What are puberty Puberty blockers are medicines that block puberty-related hormones that blockers? make your body go through puberty. Starting puberty blockers is a decision that is different for everyone. To make the most informed decision, this handout is meant to help you understand: • What is puberty? • What do puberty blockers do? • What are the changes that will happen to my body? • What are the benefits, risks and costs involved? We will work with you to support the decision that is best for you. You can view a video about puberty blockers at seattlechildrens.org/gender. How does puberty Puberty is the process the body goes through to become capable of begin? making a baby (reproduction), as well as reach adult size and brain development. Puberty starts when your brain tells your pituitary gland to start releasing puberty-related hormones. This happens at different ages for different people. During this time, your body starts to increase the amount of certain puberty-related hormones (Luteinizing Hormone (LH) and Follicle- Stimulating Hormone (FSH). This causes your testicles to start producing testosterone or your ovaries start producing estrogen. These hormones do not cause acne, pubic or armpit hair – those are caused by other hormones. Body changes in • Testicle growth (this improves the body’s ability to make testosterone) people with testicles • Penis growth (without puberty • Pubic hair blockers) • Increased acne, increased armpit and facial hair • Rapid growth (growth spurt) • Voice changes (deepens) Body changes in • Breast changes people with ovaries • Changes in body shape, including fuller hips (without puberty • Menstrual periods start (usually more than 2 years after breast changes blockers) begin) 1 of 6 To Learn More Free Interpreter Services • Adolescent Medicine • In the hospital, ask your nurse. -
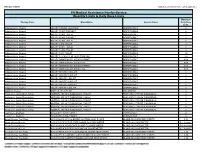
Quantity Limits/Daily Dose Limits
Effective 07/20/21 Alphabetical by Brand Name (when applicable) PA Medical Assistance Fee-for-Service Quantity Limits & Daily Dose Limits Maximum Therapy Class Brand Name Generic Name Daily Dose Limit Antipsychotics, Atypical ABILIFY 1 MG/ML SOLUTION ARIPIPRAZOLE 25 Antipsychotics, Atypical ABILIFY 10 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 10 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 15 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 15 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 2 MG TABLET ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 20 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 30 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 5 MG TABLET ARIPIPRAZOLE 1.5 Antipsychotics, Atypical ABILIFY 9.75 MG/1.3 ML INJECTION VIAL ARIPIPRAZOLE 3.9 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MYCITE 10 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 15 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 2 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 20 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 30 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 5 MG KIT ARIPIPRAZOLE 1 Antivirals, Herpes ABREVA 10% -

DEAR PHYSICIAN: This Letter Is Being Provided As a Sample to Help You with Your Payor Interactions Concerning Reimbursement
DEAR PHYSICIAN: This letter is being provided as a sample to help you with your payor interactions concerning reimbursement for the administration of SUPPRELIN® LA (histrelin acetate) subcutaneous implant. Use of this document does not guarantee coverage or reimbursement. As a healthcare professional, you are solely responsible for providing accurate information to third-party payors. If there is any information in this document that does not accurately reflect your practices, it should be modified to appropriately represent your particular circumstances. INDICATION • SUPPRELIN® LA (histrelin acetate) subcutaneous implant is indicated for the treatment of children with central precocious puberty (CPP). • Children with CPP (neurogenic or idiopathic) have an early onset of secondary sexual characteristics (earlier than 8 years of age in females and 9 years of age in males). They also show a significantly advanced bone age that can result in diminished adult height attainment. • Prior to initiation of treatment, a clinical diagnosis of CPP should be confirmed by measurement of blood concentrations of total sex steroids, luteinizing hormone (LH) and follicle stimulating hormone (FSH) following stimulation with a GnRH analog, and assessment of bone age versus chronological age. Baseline evaluations should include height and weight measurements, diagnostic imaging of the brain (to rule out intracranial tumor), pelvic/testicular/adrenal ultrasound (to rule out steroid secreting tumors), human chorionic gonadotropin levels (to rule out a chorionic gonadotropin secreting tumor), and adrenal steroids to exclude congenital adrenal hyperplasia. IMPORTANT SAFETY INFORMATION ABOUT SUPPRELIN® LA • SUPPRELIN® LA is contraindicated in patients who are hypersensitive to gonadotropin releasing hormone (GnRH) or GnRH agonist analogs and in females who are or may become pregnant while receiving the drug. -

Erleada (Apalutamide)
Market Applicability Market GA KY MD NJ NY WA Applicable X X X X X X Erleada (apalutamide) Override(s) Approval Duration Prior Authorization 1 year Quantity Limit Medications Quantity Limit Erleada (apalutamide) May be subject to quantity limit APPROVAL CRITERIA Requests for Erleada (apalutamide) may be approved if the following criteria are met: I. Individual is diagnosed with one of the following: A. Individual has a diagnosis of non-metastatic castration-resistant* prostate cancer (nmCRPC); OR B. Individual has a diagnosis of metastatic castration-sensitive prostate cancer (mCSPC) AND II. One of the following: A. Individual is concomitantly receiving a gonadotropin-releasing hormone (GnRH) analog (e.g. Lupron (leuprolide, Zoladex (goserelin), Trelstar (triptorelin), Vantas (histrelin), Firmagon (degarelix); OR B. Individual has had a bilateral orchiectomy. *Castration-resistant refers to either surgical or medically induced methods. Medically induced methods include luteinizing hormone-releasing hormone (LHRH) agonists (such as leuprolide, goserelin) or LHRH antagonists (such as degarelix). Key References: 1. Clinical Pharmacology [database online]. Tampa, FL: Gold Standard, Inc.: 2020. URL: http://www.clinicalpharmacology.com. Updated periodically. 2. DailyMed. Package inserts. U.S. National Library of Medicine, National Institutes of Health website. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed: April 19, 2020. PAGE 1 of 2 09/11/2020 CRX-ALL-0595-20 New Program Date 04/09/2018 This policy does not apply to health plans or member categories that do not have pharmacy benefits, nor does it apply to Medicare. Note that market specific restrictions or transition-of-care benefit limitations may apply. Market Applicability Market GA KY MD NJ NY WA Applicable X X X X X X 3. -

Prior Authorization Gonadotropin-Releasing Hormone Agonists Implant - Zoladex® (Goserelin Acetate Subcutaneous Implant)
Cigna National Formulary Coverage Policy Prior Authorization Gonadotropin-Releasing Hormone Agonists Implant - Zoladex® (goserelin acetate subcutaneous implant) Table of Contents Product Identifer(s) National Formulary Medical Necessity ................ 1 66292 Conditions Not Covered....................................... 2 Background .......................................................... 2 References .......................................................... 3 Revision History ................................................... 3 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s benefit plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular situation. -

Vantas (Histrelin Implant)
Vantas™ (histrelin implant) DESCRIPTION Vantas™ (histrelin implant) is a sterile non-biodegradable, diffusion-controlled reservoir drug delivery system designed to deliver histrelin continuously for 12 months upon subcutaneous implantation. The Vantas implant contains 50 mg of histrelin acetate. Histrelin acetate is a synthetic nonapeptide analogue of the naturally occurring gonadotropin releasing hormone (GnRH) or luteinizing hormone releasing hormone (LH-RH). The sterile Vantas implantation device (provided with the implant) is used to insert the implant subcutaneously in the inner aspect of the upper arm. After 12 months, the implant must be removed. At the time the implant is removed, another implant may be inserted to continue therapy. The sterile Vantas ™ implant consists of a 50-mg histrelin acetate drug core inside a nonbiodegradable,3 cm by 3.5 mm cylindrically shaped hydrogel reservoir (Figure A). The drug core also contains the inactive ingredient stearic acid NF. The hydrogel reservoir is a hydrophilic polymer cartridge composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100. The hydrated implant is packaged in a glass vial containing 2.0 mL of 1.8% NaCl solution. The implant is primed for release of the drug upon insertion. Figure A. Vantas Histrelin Implant diagram (not to scale) Histrelin acetate is chemically described as 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl Ltyrosyl-Nt-benzyl-D-histidyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) [C66H86N18O12. (1.7-2.8 moles) CH3COOH, (0.6-7.0 moles) H2O], with the molecular weight of 1443.70 (or 1323.50 as histrelin base. -

D Rug U Tilization R Eview B Oard
Wednesday, January 13, 2021 No live meeting scheduled for January. January 2021 will be a packet only meeting. Review Board Review Oklahoma Health Care Authority (OHCA) 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 Drug Utilization The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Michyla Adams, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – January 13, 2021 NOTE: No live January meeting. January 2021 is a packet only meeting. Enclosed are the following items related to the January meeting. Material is arranged in order of the agenda. Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/SoonerCare Opioid Initiative Update – Appendix B Annual Review of Gonadotropin Releasing Hormone (GnRH) Medications and 30-Day Notice to Prior Authorize Fensolvi® (Leuprolide Acetate) and Oriahnn™ (Elagolix/Estradiol/Norethindrone and Elagolix) – Appendix C Annual Review of Antihyperlipidemics and 30-Day Notice to Prior Authorize Nexletol® (Bempedoic Acid) and Nexlizet™ (Bempedoic Acid/Ezetimibe) – Appendix D Annual Review of Glaucoma Medications and 30-Day Notice to Prior Authorize Durysta™ (Bimatoprost Implant) – Appendix E Annual Review of Antiviral Medications – Appendix F Annual Review of Korlym® (Mifepristone) – Appendix G Annual Review of Turalio® (Pexidartinib) – Appendix H Annual Review of Inrebic® (Fedratinib) and Elzonris® (Tagraxofusp-erzs) – Appendix I 30-Day Notice to Prior Authorize Imcivree™ (Setmelanotide) – Appendix J U.S. Food and Drug Administration (FDA) and Drug Enforcement Administration (DEA) Updates – Appendix K Future Business ORI-4403 • P.O. BOX 26901 • OKLAHOMA CITY, OKLAHOMA 73126-0901 • (405) 271-9039 • FAX: (405) 271-2615 Oklahoma Health Care Authority Drug Utilization Review Board (DUR Board) Packet – January 13, 2021 No live January meeting. -

Supprelin LA Histrelin Prior Authorization Criteria
Prior Authorization Approval Criteria Supprelin (histrelin) Generic Name: Histrelin Brand Name: Supprelin LA®, Vantas™ Medication Class: Gonadotropin Releasing Hormone Agonist FDA Approved Uses: Vantas™ for palliative treatment of advanced prostate cancer in men Supprelin LA® for the treatment of children with central precocious puberty Available Dosage Forms: Vantas TM implantation kit 50 mg (release 50-60 mcg/day over 12 months) Supprelin ® LA implantation kit 50 mg (release~ 65 mcg/day over 12 months) Usual Dose: 50 mg implant surgically inserted every 12 months Duration of Therapy: (Vantas TM) Indefinitely (Supprelin ® LA) Until the onset of puberty (Girls ~ age 11; boys ~ age 12) Approximate monthly cost (based on ASP+6% as of 4/1/ 2008): Vantas TM has an ASP+6% of $1,508 for one implant (1 year) Supprelin® has an ASP+6% of $14,656 for one implant (1 year) Criteria for Use: (bullet points below are all inclusive unless otherwise noted) For Vantas TM for men only diagnosed with advanced prostate cancer not for children under 18 years of age For Supprelin® children age ≥ 2 years old clinically diagnosed with central precocious puberty. Diagnosis should be confirmed by the following: measurement of blood concentrations of total sex steroids (estrogens/testosterone) measurement of LH and FSH after stimulation with a GnRH analog assessment of bone age vs. chronological age Baseline evaluation should also include height and weight; diagnostic imaging of the brain, pelvic/testicular/adrenal ultrasound, HCG levels, and adrenal -

Clinical Policy: Histrelin Acetate (Vantas, Supprelin LA) Reference Number: ERX.SPMN.96 Effective Date: 07/16 Last Review Date: 06/16 Revision Log
Clinical Policy: Histrelin Acetate (Vantas, Supprelin LA) Reference Number: ERX.SPMN.96 Effective Date: 07/16 Last Review Date: 06/16 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Policy/Criteria It is the policy of health plans affiliated with Envolve Pharmacy Solutions® that histrelin acetate (Vantas® and Supprelin LA®) are medically necessary for members meeting the following criteria: I. Initial Approval Criteria A. Prostate Cancer (must meet all): 1. Age ≥ 18 years; 2. Diagnosis of advanced prostate cancer (stage T3 through T4 or high risk through nodal/metastatic disease); 3. Treatment intent is palliative; 4. Prescription is for Vantas; 5. Prescribed dose does not exceed 50 mg administered as one 12-month implant; 6. Member has a contraindication to or has failed a trial of leuprolide acetate generic injection, Lupron Depot, or Zoladex. Approval Duration: 12 months B. Central Precocious Puberty (CPP) (must meet all): 1. Females, age ≥ 2 and ≤11 years, or males, age ≥ 2 and ≤12 years; 2. Diagnosis of CPP confirmed by a through c: a. Elevated basal LH level and/or elevated leuprolide stimulated LH level >5 IU/I; b. Assessment shows significantly advanced bone age versus chronological age; c. Age at onset of secondary sex characteristics is < 8 years if female, or < 9 years if male; 3. The following conditions have been ruled out: a. Intracranial tumor with diagnostic brain imaging; b. Steroid secreting tumors with pelvic/testicular/adrenal ultrasound; c. Chorionic gonadotropin secreting tumor via measurement of human chorionic gonadotropin levels; d. Congenital adrenal hyperplasia via measurement of adrenal steroids; 4.