RX.PA.012.MPC Revision Date: 02/2020 Page Number 1 of 7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparison of the Costs for Treating Central Precocious Puberty During the First Year with Monthly Leuprolide Acetate Injectable and Histrelin Acetate Implant
A Comparison Of The Costs For Treating Central Precocious Puberty During The First Year With Monthly Leuprolide Acetate Injectable And Histrelin Acetate Implant B F Banahan III1, Mayo K2, Summers KH2 1 Center for Pharmaceutical Marketing and Management and Department of Pharmacy Administration, University of Mississippi, University, MS 2 Health Outcomes & PharmacoEconomics, Endo Pharmaceuticals, Inc., Chadds Ford, PA Estimated Annual Cost of Treatment if all PTs Treated With . BACKGROUND METHODS MEDICAID MODEL Lupron SUPPRELIN LA Product costs $ 1,770,201 $ 1,515,188 Two retrospective cohort studies were conducted using datasets derived from the Office visit costs $ 79,896 $ 14,000 Puberty results when secretion of gonadotropin releasing hormone (GnRH) is initiated 100% compliance with Lupron treatment 1 Implant procedures $ 91,400 and the hypothalamic-pituitary-gonadal axis is activated. During puberty, the brain Thomas Reuter’s MarketScan© Multi-State Medicaid Database (2003-2007) and the (All patients receive 13+ treatments in year) MarketScan© Commercial Database (2005-2009). A probabilistic patient flow model Lab/xray for monitoring $ 53,900 $ 37,500 produces GnRH through a complex process. GnRH causes increases in other TOTAL COST TO PAYER $ 1,903,997 $ 1,658,088 hormones like luteinizing hormone (LH) and follicle stimulating hormone (FSH). It is was developed using estimates for treatment patterns and costs for products, office Normal Compliance with Lupron: Lupron SUPPRELIN LA these hormones that cause the ovaries to produce estrogen and the testicles to visits, and monitoring therapy. Patients with < 13 treatments per year Product costs $ 1,572,921 $ 1,515,188 Quality of Care ofCare Quality % of TXd PTs: 53% Aver. -

Hormones and Breeding
IN-DEPTH: REPRODUCTIVE ENDOCRINOLOGY Hormones and Breeding Carlos R.F. Pinto, MedVet, PhD, Diplomate ACT Author’s address: Theriogenology and Reproductive Medicine, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, The Ohio State University, Columbus, OH 43210; e-mail: [email protected]. © 2013 AAEP. 1. Introduction affected by PGF treatment to induce estrus. In The administration of hormones to mares during other words, once luteolysis takes place, whether breeding management is an essential tool for equine induced by PGF treatment or occurring naturally, practitioners. Proper and timely administration of the events that follow (estrus behavior, ovulation specific hormones to broodmares may be targeted to and fertility) are essentially similar or minimally prevent reproductive disorders, to serve as an aid to affected (eg, decreased signs of behavioral estrus). treating reproductive disorders or hormonal imbal- Duration of diestrus and interovulatory intervals ances, and to optimize reproductive efficiency, for are shortened after PGF administration.1 The example, through induction of estrus or ovulation. equine corpus luteum (CL) is responsive to PGF These hormones, when administered exogenously, luteolytic effects any day after ovulation; however, act to control the duration and onset of the different only CL Ͼ5 days are responsive to one bolus injec- stages of the estrous cycle, specifically by affecting tion of PGF.2,3 Luteolysis or antiluteogenesis can duration of luteal function, hastening ovulation es- be reliably achieved in CL Ͻ5 days only if multiple pecially for timed artificial insemination and stimu- PGF treatments are administered. For that rea- lating myometrial activity in mares susceptible to or son, it became a widespread practice to administer showing delayed uterine clearance. -

Hertfordshire Medicines Management Committee (Hmmc) Nafarelin for Endometriosis Amber Initiation – Recommended for Restricted Use
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) NAFARELIN FOR ENDOMETRIOSIS AMBER INITIATION – RECOMMENDED FOR RESTRICTED USE Name: What it is Indication Date Decision NICE / SMC generic decision status Guidance (trade) last revised Nafarelin A potent agonistic The hormonal December Final NICE NG73 2mg/ml analogue of management of 2020 Nasal Spray gonadotrophin endometriosis, (Synarel®) releasing hormone including pain relief and (GnRH) reduction of endometriotic lesions HMMC recommendation: Amber initiation across Hertfordshire (i.e. suitable for primary care prescribing after specialist initiation) as an option in endometriosis Background Information: Gonadorelin analogues (or gonadotrophin-releasing hormone agonists [GnRHas]) include buserelin, goserelin, leuprorelin, nafarelin and triptorelin. The current HMMC decision recommends triptorelin as Decapeptyl SR® injection as the gonadorelin analogue of choice within licensed indications (which include endometriosis) link to decision. A request was made by ENHT to use nafarelin nasal spray as an alternative to triptorelin intramuscular injection during the COVID-19 pandemic. The hospital would provide initial 1 month supply, then GPs would continue for further 5 months as an alternative to the patient attending for further clinic appointments for administration of triptorelin. Previously at ENHT, triptorelin was the only gonadorelin analogue on formulary for gynaecological indications. At WHHT buserelin nasal spray 150mcg/dose is RED (hospital only) for infertility & endometriosis indications. Nafarelin nasal spray 2mg/ml is licensed for: . The hormonal management of endometriosis, including pain relief and reduction of endometriotic lesions. Use in controlled ovarian stimulation programmes prior to in-vitro fertilisation, under the supervision of an infertility specialist. Use of nafarelin in endometriosis aims to induce chronic pituitary desensitisation, which gives a menopause-like state maintained over many months. -

Degarelix for Treating Advanced Hormone- Dependent Prostate Cancer
CONFIDENTIAL UNTIL PUBLISHED NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Final appraisal determination Degarelix for treating advanced hormone- dependent prostate cancer This guidance was developed using the single technology appraisal (STA) process 1 Guidance 1.1 Degarelix is recommended as an option for treating advanced hormone-dependent prostate cancer, only in adults with spinal metastases who present with signs or symptoms of spinal cord compression. 1.2 People currently receiving treatment initiated within the NHS with degarelix that is not recommended for them by NICE in this guidance should be able to continue treatment until they and their NHS clinician consider it appropriate to stop. 2 The technology 2.1 Degarelix (Firmagon, Ferring Pharmaceuticals) is a selective gonadotrophin-releasing hormone antagonist that reduces the release of gonadotrophins by the pituitary, which in turn reduces the secretion of testosterone by the testes. Gonadotrophin- releasing hormone is also known as luteinising hormone-releasing hormone (LHRH). Because gonadotrophin-releasing hormone antagonists do not produce a rise in hormone levels at the start of treatment, there is no initial testosterone surge or tumour stimulation, and therefore no potential for symptomatic flares. National Institute for Health and Care Excellence Page 1 of 71 Final appraisal determination – Degarelix for treating advanced hormone-dependent prostate cancer Issue date: April 2014 CONFIDENTIAL UNTIL PUBLISHED Degarelix has a UK marketing authorisation for the ‘treatment of adult male patients with advanced hormone-dependent prostate cancer’. It is administered as a subcutaneous injection. 2.2 The most common adverse reactions with degarelix are related to the effects of testosterone suppression, including hot flushes and weight increase, or injection site reactions (such as pain and erythema). -

The Use of an Intravaginal Triptorelin Gel to Induce Ovulation in the Mare
THE USE OF AN INTRAVAGINAL TRIPTORELIN GEL TO INDUCE OVULATION IN THE MARE by CHELSEA D. SINCLAIR B.S., Clemson University, 2013 A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Animal Sciences and Industry College of Agriculture KANSAS STATE UNIVERSITY Manhattan, Kansas 2016 Approved by: Major Professor Dr. Joann M. Kouba Copyright CHELSEA D. SINCLAIR 2016 Abstract The objective of these studies was to investigate the efficacy of an intravaginal triptorelin acetate (TA) gel as an ovulation-inducing agent in mares. In Exp 1, 24 mares were stratified by parity and age and randomly assigned to 3 treatment groups receiving either: 5 mL TA gel (500 μg TA; TA5), 10 mL TA gel (1,000 μg TA; TA10), or 5 mL vehicle gel only (CON). Following the appearance of a follicle ≥ 25 mm, blood samples were obtained every 24 h until treatment administration for measurement of luteinizing hormone (LH) concentrations. Once a follicle ≥ 35 mm in diameter was detected, treatment was administered intravaginally. Following treatment, blood samples were collected and ovaries were scanned via transrectal ultrasonography every 12 h until 48 h post-ovulation. Both TA5 and TA10 tended (P = 0.08) to experience a brief surge in LH by 12 h post-treatment. Regarding LH concentrations, there was a significant (P < 0.005) treatment by time interaction. The interval from treatment to ovulation was not different (P > 0.05) between groups, nor was there a difference (P > 0.05) in the percentage of mares ovulating within 48 h of treatment administration. -

Triptorelin, Goserelin and Leuprorelin for Prostate
789FM.1 GONADORELIN ANALOGUES (GnRHa): TRIPTORELIN, GOSERELIN, LEUPRORELIN FOR PROSTATE CANCER - AMBER RECOMMENDATION GUIDELINE This guideline provides prescribing and monitoring guidance for triptorelin, goserelin and Leuprorelin use in prostate cancer. It should be read in conjunction with the Summary of Product Characteristics (SPC), available on www.medicines.org.uk/emc, and the BNF. BACKGROUND AND INDICATIONS FOR USE There is no conclusive evidence to suggest that any one GnRHa is more effective or has fewer side effects than another for the treatment of prostate cancer.5, 7-9 Available evidence suggests that GnRHa are similar in effectiveness to surgical castration. They have the same licensed indications in the treatment of prostate cancer (Appendix 1). Appendix 2 compares doses, administration frequency and costs.1-4, 9-13 Triptorelin 22.5 mg (six monthly injection) is the 1st choice GnRHa for treatment of metastatic prostate cancer patients on life-long treatment on the Bucks formulary. This is because it is: • Administered via a smaller sized needle (20 gauge) compared to goserelin LA 10.8 mg (14 gauge), thus minimising discomfort to patients.9 • The least expensive in terms of drug and administration costs (Appendix 2).1-4, 9-13 Triptorelin 22.5 mg (six monthly injection) is not preferred in: • Patients newly initiated GnRHa because the first dose should be a monthly preparation (Decapeptyl® SR 3 mg) in order to check that the product is tolerated prior to changing to the six monthly preparation.14 • Anticoagulated patients. This is because triptorelin is an intramuscular (IM) injection. Subcutaneously administered GnRHa (goserelin or leuprorelin) may be preferable.9 RESPONSIBILITIES Hospital specialist 1. -

DESCRIPTION Vantas™ (Histrelin Implant) Is a Sterile Non-Biodegradable, Diffusion-Controlled Reservoir Drug Delivery System De
Vantas™ (histrelin implant) PACKAGE INSERT DESCRIPTION Vantas™ (histrelin implant) is a sterile non-biodegradable, diffusion-controlled reservoir drug delivery system designed to deliver histrelin continuously for 12 months upon subcutaneous implantation. The Vantas implant contains 50 mg of histrelin acetate. Histrelin acetate is a synthetic nonapeptide analogue of the naturally occurring gonadotropin releasing hormone (GnRH) or luteinizing hormone releasing hormone (LH-RH). The sterile Vantas implantation device (provided with the implant) is used to insert the implant subcutaneously in the inner aspect of the upper arm. After 12 months, the implant must be removed. At the time the implant is removed, another implant may be inserted to continue therapy. The sterile Vantas ™ implant consists of a 50-mg histrelin acetate drug core inside a non- biodegradable, 3 cm by 3.5 mm cylindrically shaped hydrogel reservoir (Figure A). The drug core also contains the inactive ingredient stearic acid NF. The hydrogel reservoir is a hydrophilic polymer cartridge composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100. The hydrated implant is packaged in a glass vial containing 2.0 mL of 1.8% NaCl solution. The implant is primed for release of the drug upon insertion. Figure A. Vantas Histrelin Implant diagram (not to scale) Hydrogel Reservoir Drug Formulation Histrelin acetate is chemically described as 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L- tyrosyl-Nt-benzyl-D-histidyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) [C66H86N18O12. (1.7-2.8 moles) CH3COOH, (0.6-7.0 moles) H2O], with the molecular weight of 1443.70 (or 1323.50 as histrelin base. -

Comparison of Oocyte Maturity Rates in Recombinant Human Chorionic Go
International Journal of ISSN 2692-5877 Clinical Studies & Medical Case Reports DOI: 10.46998/IJCMCR.2020.07.000150 Research Article Comparison of Oocyte Maturity Rates in Recombinant Human Chorionic Go- nadotrophin (HCG) and Triptorelin Acetate Triggers; A Prospective Randomised Study Lakshmanan S1, Saravanan M1, Senthil P1* and Sharma N2 1ARC International Fertility Center, 947, Avinashi Road, Puliakulam, Coimbatore, 641037, Tamilnadu, India 2Department of Obstetrics and Gynecology, Saveetha University, Kuthambaakam, Chennai-600124, Tamil Nadu, India *Corresponding author: Priya Senthil, ARC International Fertility Center, 947, Avinashi Road, Puliakulam, Coim- batore, 641037, Tamilnadu, India. Tel: 8220812314; E-mail: [email protected] Received: September 16, 2020 Published:November 20, 2020 Abstract LH like exposure in the mid cycle for inducing the oocyte maturation, is the very crucial step in the success of ICSI treatment. Introduction of LH surge endogenously by GnRH-agonist for final oocyte maturation induction, may be more physiological compared with the administration of HCG. Since GnRH agonist would induce FSH surge also along with LH surge, as happens in natural cycle. However, the effects of giving HCG trigger for inducing only LH surge and giv- ing GnRH agonist trigger for inducing both LH and FSH surge, in patients treated for ICSI with GnRHantagonists need more research. Sub fertile patients planned for ICSI, meeting the requirement of inclusion criteria, were started with re- combinant FSH from day 2 of menstrual cycle. GnRH antagonists were started from day 6 of stimulation. FSH dose was adjusted according to the individual response. Trigger was planned when the lead follicle reaches 24 mm. For triggering, 100 patients were randomised to receive Recombinant HCG trigger and Triptorelin acetate trigger. -

PE2572 Puberty Blockers
Puberty Blockers What are puberty Puberty blockers are medicines that block puberty-related hormones that blockers? make your body go through puberty. Starting puberty blockers is a decision that is different for everyone. To make the most informed decision, this handout is meant to help you understand: • What is puberty? • What do puberty blockers do? • What are the changes that will happen to my body? • What are the benefits, risks and costs involved? We will work with you to support the decision that is best for you. You can view a video about puberty blockers at seattlechildrens.org/gender. How does puberty Puberty is the process the body goes through to become capable of begin? making a baby (reproduction), as well as reach adult size and brain development. Puberty starts when your brain tells your pituitary gland to start releasing puberty-related hormones. This happens at different ages for different people. During this time, your body starts to increase the amount of certain puberty-related hormones (Luteinizing Hormone (LH) and Follicle- Stimulating Hormone (FSH). This causes your testicles to start producing testosterone or your ovaries start producing estrogen. These hormones do not cause acne, pubic or armpit hair – those are caused by other hormones. Body changes in • Testicle growth (this improves the body’s ability to make testosterone) people with testicles • Penis growth (without puberty • Pubic hair blockers) • Increased acne, increased armpit and facial hair • Rapid growth (growth spurt) • Voice changes (deepens) Body changes in • Breast changes people with ovaries • Changes in body shape, including fuller hips (without puberty • Menstrual periods start (usually more than 2 years after breast changes blockers) begin) 1 of 6 To Learn More Free Interpreter Services • Adolescent Medicine • In the hospital, ask your nurse. -

Triptodur (Triptorelin)
Triptodur (triptorelin) (Intramuscular) Document Number: IC-0308 Last Review Date: 08/04/2020 Date of Origin: 08/01/2017 Dates Reviewed: 08/2017, 08/2018, 08/2019, 08/2020 I. Length of Authorization Coverage will be provided for 12 months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Units]: • Triptodur 22.5 mg single-use kit: 1 kit per 168 days B. Max Units (per dose and over time) [HCPCS Units]: • 6 billable units per 168 days III. Initial Approval Criteria Coverage is provided in the following conditions: Central Precocious Puberty (CPP) † Ф 1,2-4,5 • Patient is between the ages of 2 and 13 years; AND • Onset of secondary sexual characteristics earlier than age 8 for girls and 9 for boys associated with pubertal pituitary gonadotropin activation; AND • Diagnosis is confirmed by pubertal gonadal sex steroid levels and a pubertal LH response to stimulation by native GnRH; AND • Bone age advanced greater than 2 standard deviations (SD) beyond chronological age; AND • Tumor has been ruled out by lab tests such as diagnostic imaging of the brain (to rule out intracranial tumor), pelvic/testicular/adrenal ultrasound (to rule out steroid secreting tumors), and human chorionic gonadotropin levels (to rule out a chorionic gonadotropin secreting tumor). † FDA Approved Indication(s); ‡ Compendia Recommended Indication(s); Ф Orphan Drug Proprietary & Confidential © 2020 Magellan Health, Inc. 1 IV. Renewal Criteria Authorizations can be renewed based on the following criteria: • Patient continues to meet indication-specific relevant criteria identified in section III; ; AND • Absence of unacceptable toxicity from the drug. -

Neoadjuvant Degarelix Versus Triptorelin in Premenopausal
original report Neoadjuvant Degarelix Versus Triptorelin in Premenopausal Patients Who Receive Letrozole for Locally Advanced Endocrine-Responsive Breast Cancer: A Randomized Phase II Trial Silvia Dellapasqua, MD1; Kathryn P. Gray, PhD2; Elisabetta Munzone, MD1; Daniela Rubino, MD3; Lorenzo Gianni, MD4; Harriet Johansson, PhD, MSc1; Giuseppe Viale, MD1;KarinRibi,PhD,MPH5;Jurg¨ Bernhard, PhD5; Roswitha Kammler5; Rudolf Maibach, PhD5; Manuela Rabaglio-Poretti, MD5; Barbara Ruepp, PharmD5; Angelo Di Leo, MD, PhD6;AlanS.Coates,MD7; Richard D. Gelber, PhD8,9; Meredith M. Regan, ScD9; Aron Goldhirsch, MD1; and Marco Colleoni, MD1, for the International Breast Cancer Study Group abstract PURPOSE To evaluate endocrine activity in terms of ovarian function suppression (OFS) of degarelix (a gonadotropin-releasing hormone [GnRH] antagonist) versus triptorelin (a GnRH agonist) in premenopausal patients receiving letrozole as neoadjuvant endocrine therapy for breast cancer. PATIENTS AND METHODS Premenopausal women with stage cT2 to 4b, any N, M0; estrogen receptor and progesterone receptor greater than 50%; human epidermal growth factor receptor 2–negative breast cancer were randomly assigned to triptorelin 3.75 mg administered intramuscularly on day 1 of every cycle or degarelix 240 mg administered subcutaneously (SC) on day 1 of cycle 1 then 80 mg SC on day 1 of cycles 2 through 6, both with letrozole 2.5 mg/day for six 28-day cycles. Surgery was performed 2 to 3 weeks after the last injection. ASSOCIATED Serum was collected at baseline, after 24 and 72 hours, at 7 and 14 days, and then before injections on cycles 2 CONTENT through 6. The primary end point was time to optimal OFS (time from the first injection to first assessment of Appendix centrally assessed estradiol level # 2.72 pg/mL [# 10 pmol/L] during neoadjuvant therapy). -
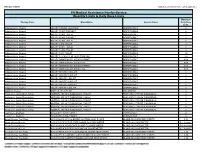
Quantity Limits/Daily Dose Limits
Effective 07/20/21 Alphabetical by Brand Name (when applicable) PA Medical Assistance Fee-for-Service Quantity Limits & Daily Dose Limits Maximum Therapy Class Brand Name Generic Name Daily Dose Limit Antipsychotics, Atypical ABILIFY 1 MG/ML SOLUTION ARIPIPRAZOLE 25 Antipsychotics, Atypical ABILIFY 10 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 10 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 15 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 15 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 2 MG TABLET ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 20 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 30 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 5 MG TABLET ARIPIPRAZOLE 1.5 Antipsychotics, Atypical ABILIFY 9.75 MG/1.3 ML INJECTION VIAL ARIPIPRAZOLE 3.9 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MYCITE 10 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 15 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 2 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 20 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 30 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 5 MG KIT ARIPIPRAZOLE 1 Antivirals, Herpes ABREVA 10%