Response of Water Quality and the Aquatic Community to a Reverse Osmosis Unit Discharge Into the Wichita River, Texas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020 Draft Basin Highlights Report an Overview of Water Quality Issues Throughout the Canadian and Red River Basins
2020 DRAFT BASIN HIGHLIGHTS REPORT AN OVERVIEW OF WATER QUALITY ISSUES THROUGHOUT THE CANADIAN AND RED RIVER BASINS The preparation of this report was financed through and in cooperation with the Texas Commission on Environmental Quality North Fork Red River at FM 2473 2020 Canadian and Red River Basins Highlights Report ~ Page 2020 Canadian and Red River Basins Highlights Report ~ Page 2 Lake Texoma at US 377 Bridge TABLE OF CONTENTS CANADIAN AND RED RIVER BASIN VICINITY MAP 4 INTRODUCTION 5 Public Involvement Basin Advisory Committee Meeting 6 Coordinated Monitoring Meeting 7 Zebra Mussels Origin, Transportation, Impact, Texas Bound, Current Populations, and Studies 8 Texas Legislation Action 9 CANADIAN AND RED RIVER BASINS WATER QUALITY OVERVIEW AND HIGHLIGHTS Canadian and Red River Basins Water Quality Overview, 2018 Texas IR Overview 10 TABLES Canadian River Basin 2018 Texas IR Impairment Listing 11 Red River Basin 2018 Texas IR Impairment Listing 12 Water Quality Monitoring Field Parameters, Conventional Laboratory Parameters Red River Authority Environmental Services Laboratory Environmental Services Division 15 2020 Canadian and Red River Basins Highlights Report ~ Page 3 2020 Canadian and Red River Basins Highlights Report ~ Page 4 INTRODUCTION In 1991, the Texas Legislature enacted the Texas Clean Rivers Act (Senate Bill 818) in order to assess water quality for each river basin in the state. From this, the Clean Rivers Program (CRP) was created and has become one of the most successful cooperative efforts between federal, state, and local agen- cies and the citizens of the State of Texas. It is implemented by the Texas Commission on Environ- mental Quality (TCEQ) through local partner agencies to achieve the CRP’s primary goal of maintain- ing and improving the water quality in each river basin. -

Classified Stream Segments and Assessments Units Covered by Hb 4146
CLASSIFIED STREAM SEGMENTS AND ASSESSMENTS UNITS COVERED BY HB 4146 SEG ID River Basin Description Met criteria 0216 Red Wichita River Below Lake Kemp Dam 96.43% 0222 Red Salt Fork Red River 95.24% 0224 Red North Fork Red River 90.91% 1250 Brazos South Fork San Gabriel River 93.75% 1251 Brazos North Fork San Gabriel River 93.55% 1257 Brazos Brazos River Below Lake Whitney 90.63% 1415 Colorado Llano River 94.39% 1424 Colorado Middle Concho/South Concho River 95.24% 1427 Colorado Onion Creek 93.43% 1430 Colorado Barton Creek 98.25% 1806 Guadalupe Guadalupe River Above Canyon Lake 96.37% 1809 Guadalupe Lower Blanco River 95.83% 1811 Guadalupe Comal River 98.90% 1812 Guadalupe Guadalupe River Below Canyon Dam 96.98% 1813 Guadalupe Upper Blanco River 95.45% 1815 Guadalupe Cypress Creek 99.19% 1816 Guadalupe Johnson Creek 97.30% AU ID River Basin Description Met criteria 1817 Guadalupe North Fork Guadalupe River 100.00% 1414_01 Colorado Pedernales River 93.10% 1818 Guadalupe South Fork Guadalupe River 97.30% 1414_03 Colorado Pedernales River 91.38% 1905 San Antonio Medina River Above Medina Lake 100.00% 1416_05 Colorado San Saba River 100.00% 2111 Nueces Upper Sabinal River 100.00% Colorado River Below Lady Bird Lake 2112 Nueces Upper Nueces River 95.96% 1428_03 Colorado (formally Town Lake) 91.67% 2113 Nueces Upper Frio River 100.00% Medina River Below Medina 1903_04 San Antonio Diversion Lake 90.00% 2114 Nueces Hondo Creek 93.48% Medina River Below Medina 2115 Nueces Seco Creek 95.65% 1903_05 San Antonio Diversion Lake 96.84% 2309 Rio Grande Devils River 96.67% 1908_02 San Antonio Upper Cibolo Creek 97.67% 2310 Rio Grande Lower Pecos River 93.88% 2304_10 Rio Grande Rio Grande Below Amistad Reservoir 95.95% 2313 Rio Grande San Felipe Creek 95.45% 2311_01 Rio Grande Upper Pecos River 92.86% A detailed map of covered segments and assessment units can be found on the TCEQ website https://www.tceq.texas.gov/gis/nonpoint-source-project-viewer . -

United States Department of the Interior National Park Service Land
United States Department of the Interior National Park Service Land & Water Conservation Fund --- Detailed Listing of Grants Grouped by County --- Today's Date: 11/20/2008 Page: 1 Texas - 48 Grant ID & Type Grant Element Title Grant Sponsor Amount Status Date Exp. Date Cong. Element Approved District ANDERSON 396 - XXX D PALESTINE PICNIC AND CAMPING PARK CITY OF PALESTINE $136,086.77 C 8/23/1976 3/1/1979 2 719 - XXX D COMMUNITY FOREST PARK CITY OF PALESTINE $275,500.00 C 8/23/1979 8/31/1985 2 ANDERSON County Total: $411,586.77 County Count: 2 ANDREWS 931 - XXX D ANDREWS MUNICIPAL POOL CITY OF ANDREWS $237,711.00 C 12/6/1984 12/1/1989 19 ANDREWS County Total: $237,711.00 County Count: 1 ANGELINA 19 - XXX C DIBOLL CITY PARK CITY OF DIBOLL $174,500.00 C 10/7/1967 10/1/1971 2 215 - XXX A COUSINS LAND PARK CITY OF LUFKIN $113,406.73 C 8/4/1972 6/1/1973 2 297 - XXX D LUFKIN PARKS IMPROVEMENTS CITY OF LUFKIN $49,945.00 C 11/29/1973 1/1/1977 2 512 - XXX D MORRIS FRANK PARK CITY OF LUFKIN $236,249.00 C 5/20/1977 1/1/1980 2 669 - XXX D OLD ORCHARD PARK CITY OF DIBOLL $235,066.00 C 12/5/1978 12/15/1983 2 770 - XXX D LUFKIN TENNIS IMPROVEMENTS CITY OF LUFKIN $51,211.42 C 6/30/1980 6/1/1985 2 879 - XXX D HUNTINGTON CITY PARK CITY OF HUNTINGTON $35,313.56 C 9/26/1983 9/1/1988 2 ANGELINA County Total: $895,691.71 County Count: 7 United States Department of the Interior National Park Service Land & Water Conservation Fund --- Detailed Listing of Grants Grouped by County --- Today's Date: 11/20/2008 Page: 2 Texas - 48 Grant ID & Type Grant Element Title Grant Sponsor Amount Status Date Exp. -
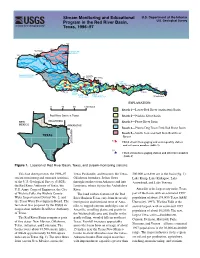
Stream Monitoring and Educational Program in the Red River Basin
Stream Monitoring and Educational U.S. Department of the Interior Program in the Red River Basin, U.S. Geological Survey Texas, 1996–97 100 o 101 o 5 AMARILLO NORTH FORK 102 o RED RIVER 103 o A S LT 35o F ORK RED R IV ER 1 4 2 PRAIRIE DOG TOWN PEASE 3 99 o WICHITA FORK RED RIVER 7 FALLS CHARLIE 6 RIVE R o o 34 W 8 98 9 I R o LAKE CHIT 21 ED 97 A . TEXOMA o VE o 10 11 R 25 96 RI R 95 16 19 18 20 DENISON 17 28 14 15 23 24 27 29 22 26 30 12,13 LAKE PARIS KEMP LAKE LAKE KICKAPOO ARROWHEAD TEXARKANA EXPLANATION 0 40 80 120 MILES Reach 1—Lower Red River (mainstem) Basin Red River Basin in Texas Reach 2—Wichita River Basin NEW OKLAHOMA Reach 3—Pease River Basin MEXICO ARKANSAS Reach 4—Prairie Dog Town Fork Red River Basin Reach 5—North Fork and Salt Fork Red River TEXAS Basins 12 LOUISIANA USGS streamflow-gaging and water-quality station and reference number (table 1) 22 USGS streamflow-gaging station and reference number (table 1) Figure 1. Location of Red River Basin, Texas, and stream-monitoring stations. This fact sheet presents the 1996–97 Texas Panhandle, and becomes the Texas- 200,000 acre-feet are in the basin (fig. 1): stream monitoring and outreach activities Oklahoma boundary. It then flows Lake Kemp, Lake Kickapoo, Lake of the U.S. Geological Survey (USGS), through southwestern Arkansas and into Arrowhead, and Lake Texoma. -

Index of Surface Water Stations in Texas
1 UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY I AUSTIN, TEXAS INDEX OF SURFACE WATER STATIONS IN TEXAS Operated by the Water Resources Division of the Geological Survey in cooperation with State and Federal Agencies Gaging Station 08065000. Trinity River near Oakwood , October 1970 UNITED STATES DEPARTMENT OF THE INTERIOR Geological Survey - Water Resources Division INDEX OF SURFACE WATER STATIONS IN TEXAS OCTOBER 1970 Copies of this report may be obtained from District Chief. Water Resources Division U.S. Geological Survey Federal Building Austin. Texas 78701 1970 CONTENTS Page Introduction ............................... ................•.......•...•..... Location of offices .........................................•..•.......... Description of stations................................................... 2 Definition of tenns........... • . 2 ILLUSTRATIONS Location of active gaging stations in Texas, October 1970 .•.•.•.••..•••••..•.. 1n pocket TABLES Table 1. Streamflow, quality, and reservoir-content stations •.•.•... ~........ 3 2. Low-fla.o~ partial-record stations.................................... 18 3. Crest-stage partial-record stations................................. 22 4. Miscellaneous sites................................................. 27 5. Tide-level stations........................ ........................ 28 ii INDEX OF SURFACE WATER STATIONS IN TEXAS OCTOBER 1970 The U.S. Geological Survey's investigations of the water resources of Texas are con ducted in cooperation with the Texas Water Development -

Stormwater Management Program 2013-2018 Appendix A
Appendix A 2012 Texas Integrated Report - Texas 303(d) List (Category 5) 2012 Texas Integrated Report - Texas 303(d) List (Category 5) As required under Sections 303(d) and 304(a) of the federal Clean Water Act, this list identifies the water bodies in or bordering Texas for which effluent limitations are not stringent enough to implement water quality standards, and for which the associated pollutants are suitable for measurement by maximum daily load. In addition, the TCEQ also develops a schedule identifying Total Maximum Daily Loads (TMDLs) that will be initiated in the next two years for priority impaired waters. Issuance of permits to discharge into 303(d)-listed water bodies is described in the TCEQ regulatory guidance document Procedures to Implement the Texas Surface Water Quality Standards (January 2003, RG-194). Impairments are limited to the geographic area described by the Assessment Unit and identified with a six or seven-digit AU_ID. A TMDL for each impaired parameter will be developed to allocate pollutant loads from contributing sources that affect the parameter of concern in each Assessment Unit. The TMDL will be identified and counted using a six or seven-digit AU_ID. Water Quality permits that are issued before a TMDL is approved will not increase pollutant loading that would contribute to the impairment identified for the Assessment Unit. Explanation of Column Headings SegID and Name: The unique identifier (SegID), segment name, and location of the water body. The SegID may be one of two types of numbers. The first type is a classified segment number (4 digits, e.g., 0218), as defined in Appendix A of the Texas Surface Water Quality Standards (TSWQS). -

Index of Surface-Water Stations in Texas, January 1989
INDEX OF SURFACE-WATER STATIONS IN TEXAS, JANUARY 1989 Compiled by Jack Rawson, E.R. Carrillo, and H.D. Buckner U.S. GEOLOGICAL SURVEY Open-File Report 89-265 Austin, Texas 1989 DEPARTMENT OF THE INTERIOR DONALD PAUL MODEL, Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director For additional information Copies of this report can write to: be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Books and Open-File Reports 8011 Cameron Road Federal Center, Bldg. 810 Austin, Texas 78753 Box 25425 Denver, Colorado 80225 CONTENTS Page Introduction.......................................................... 1 Definition of terms................................................... 2 Availability of data .................................................. 3 ILLUSTRATIONS Plate 1. Map showing the locations of active surface-water stations in Texas, January 1989.................................... In pocket 2. Map showing the locations of active partia1-record surface- water stations in Texas, January 1989..................... In pocket TABLES Table 1. Streamflow, quality, reservoir-content, and partial-record stations maintained by the U.S. Geological Survey in cooperation with State and Federal agencies............... 5 111 INDEX OF SURFACE-WATER STATIONS IN TEXAS JANUARY 1989 Compi1ed by Jack Rawson, E.R. Carrillo, and H.D. Buckner INTRODUCTION The U.S. Geological Survey's investigations of the water resources of Texas are conducted in cooperation with the Texas Water Development Board, river authorities, cities, counties, U.S. Army Corps of Engineers, U.S. Bureau of Reclamation, Interna tional Boundary and Water Commission, and others. Investigations are under the general direction of C.W. Boning, District Chief, Texas District. The Texas District office is located at 8011A Cameron Road, Austin, Texas 78753. -

Curriculum Vitae
CURRICULUM VITAE Jenny Wrast Oakley 2700 Bay Area Blvd. | MC 540 | Houston, TX 77058 [email protected] | (281) 283-3947 www.eih.uhcl.edu EDUCATION Ph.D. Marine Biology Interdisciplinary Program, Texas A&M University, 2019 Dissertation: “Coastal Health Index for the Northern Gulf of Mexico: Moving towards Meaningful Ecosystem-Based Management.” M.S. Biology, Texas A&M University-Corpus Christi, 2008 Thesis: “Spatial and Temporal Variability in Food Web Structure on Subtidal Oyster Reefs in Lavaca Bay, Texas.” B.S. Biology, Texas A&M University-Corpus Christi, 2006 Minor in Chemistry. PROFESSIONAL BACKGROUND Adjunct Faculty January 2021-Present College of Science and Engineering, University of Houston-Clear Lake, Houston TX -Instruct 1 section of Conservation Biology (BIOL 5534) Associate Director, Research Programs December 2020-Present Environmental Institute of Houston, University of Houston-Clear Lake, Houston TX -Manage EIH Environmental Research Department -Development and implementation of research budget -Responsible for research program policy, long-range forecasting, and strategic planning -Supervise research grants: scheduling, study design, data analysis, training and quality assurance Environmental Scientist September 2010-December 2020 Environmental Institute of Houston, University of Houston-Clear Lake, Houston TX -Supervise EIH Environmental Research Department -Grant and proposal writing -Data Analysis and final report writing Graduate Assistant Non-Teaching (GANT) January 2015-May 2015 Texas A&M University, College Station, -

Floods in South-Central Oklahoma and North-Central Texas October 1981
FLOODS IN SOUTH-CENTRAL OKLAHOMA AND NORTH-CENTRAL TEXAS OCTOBER 1981 By Harold D. Buckner and Joanne K. Kurklin U.S. GEOLOGICAL SURVEY Open-File Report 84-065 Austin, Texas 1984 UNITED STATES DEPARTMENT OF THE INTERIOR WILLIAM P. CLARK, Secretary GEOLOGICAL SURVEY Dallas L. Peck, Director For additional information For sale by: write to: District Chief Open-File Services Section U.S. Geological Survey Western Distribution Branch 649 Federal Building U.S. Geological Survey, MS 306 300 E. Eighth Street Box 25425, Denver Federal Center Austin, TX 78701 Denver, CO 80225 Telephone: (303) 234-5888 II CONTENTS Page Abstract 1 Introduction- 2 Meteorological setting and precipitation distribution 4 Description of floods- 7 Red River basin 20 Trinity River basin- 25 Brazos River basin 28 Flood damages 33 Oklahoma 33 Texas- 33 Explanation of station data 36 References cited- 37 Supplementary data 38 III ILLUSTRATIONS Page Figure 1. Map showing area of flooding in Oklahoma and Texas with location of flood-determination points 3 2. Map showing surface front, upper level trough line, and jet- stream on October 11, 1981 5 3. Map showing surface front, upper level trough line, outflow boundary, jetstream, and path of Hurricane Norrna- 6 4a-f. GOES enhanced infrared and visual imagery pictures showing track of Hurricane Norma across Mexico and Texas: a. 1:30 a.m. c.d.t., October 12, 1981 8 b. 5:00 a.m. c.d.t., October 12, 1981 9 c. 9:30 a.m. c.d.t., October 12, 1981 10 d. 1:30 p.m. c.d.t., October 12, 1981 11 e. -

Little Rock, Arkansas
LITTLE ROCK, ARKANSAS The civil works portion of this District covers an area of the District is responsible for the portion of the Little approximately 36,414 square miles in northern, western, River and its tributaries that are in the state of Arkansas, and southwestern Arkansas and a portion of Missouri. above its mouth near Fulton, AR. In the White River This area is within the Arkansas River, Little River, and Basin, the District is responsible for those portions in White River basins. In the Arkansas River Basin, the southern Missouri and northern and eastern Arkansas in District is responsible for planning, design, construction, the White River drainage basin and its tributaries above operation, and maintenance of the navigation portion of Peach Orchard Bluff, AR. The Memphis District is re- the McClellan-Kerr Arkansas River Navigation System sponsible for navigation maintenance on the White River (MKARNS). The District is also responsible for the below Newport, AR, to the mouth of Wild Goose areas included in the Arkansas River drainage basin Bayou, in Arkansas County, AR. The White River from above Pine Bluff, AR, to below the mouth of the downstream from the mouth of Wild Goose Bayou is Poteau River, near Fort Smith, AR. In Little River Basin, part of the MKARNS. IMPROVEMENTS NAVIGATION Multiple-Purpose Projects Including Power 1. Arkansas River Basin, AR, OK, And KS ........... 3 2. Arthur V. Ormond Lock & Dam (No.9), AR ..... 3 28. Beaver Lake, AR ………………………………9 3. David D. Terry Lock And Dam (No. 6), AR ...... 4 29. Bull Shoals Lake, AR.................................. ….10 4. -

Distributional Surveys of Freshwater Bivalves in Texas: Progress Report for 1997
DISTRIBUTIONAL SURVEYS OF FRESHWATER BIVALVES IN TEXAS: PROGRESS REPORT FOR 1997 by Robert G. Howells MANAGEMENT DATA SERIES No. 147 1998 Texas Parks and Wildlife Depar1ment Inland Fisheries Division 4200 Smith School Road Austin, Texas 78744 ACKNOWLEDGMENTS Many biologists and technicians with Texas Parks and Wildlife Department's Inland Fisheries Research and Management offices assisted with SW"Veys and collectons of freshwater mussels. Thanks also go to Pam Balcer (Kerrville, Texas) and Sue Martin (San Angelo; Texas) who assised extensively with collection of specimens and Jesse Todd (Dallas, Texas), Dr. Charles Mather (University of Arts and Science, Chickasha, Oklahoma) and J.A.M. Bergmann (Boerne, Texas) who provided specimens and field data. ABSTRACT During 1997, over 1,500 unionid specimens were docwnented from 87 locations (I 06 sample sites) statewide in Texas where specimens were either directly surveyed by the Heart of the Hills Research Station (HOH) staff or were sent to HOH by volunteers. Living specimens or recently-dead shells were found at 59% of the locations, 14% yielded only Jong-dead or subfossil shells, 24% produced no unionids or their remains, and 3% could not be accessed due to private lands or other local site problems which precluded sampling. Jn conjunction with previous field-survey work J 992-1996, unionids appear completely or almost completely extirpated from the Pedernales, Blanco, San Marcos, Llano, Medina, upper Guadalupe, upper Sulphur, areas of the San Jacinto, and much of the San Saba rivers. Sections of other river systems and many tributaries have also experienced major unionid population losses in recent years. -

Figure: 30 TAC §307.10(1) Appendix A
Figure: 30 TAC §307.10(1) Appendix A - Site-specific Uses and Criteria for Classified Segments The following tables identify the water uses and supporting numerical criteria for each of the state's classified segments. The tables are ordered by basin with the segment number and segment name given for each classified segment. Marine segments are those that are specifically titled as "tidal" in the segment name, plus all bays, estuaries and the Gulf of Mexico. The following descriptions denote how each numerical criterion is used subject to the provisions in §307.7 of this title (relating to Site-Specific Uses and Criteria), §307.8 of this title (relating to Application of Standards), and §307.9 of this title (relating to Determination of Standards Attainment). Segments that include reaches that are dominated by springflow are footnoted in this appendix and have critical low-flows calculated according to §307.8(a)(2) of this title. These critical low-flows apply at or downstream of the spring(s) providing the flows. Critical low-flows upstream of these springs may be considerably smaller. Critical low-flows used in conjunction with the Texas Commission on Environmental Quality regulatory actions (such as discharge permits) may be adjusted based on the relative location of a discharge to a gauging station. -1 -2 The criteria for Cl (chloride), SO4 (sulfate), and TDS (total dissolved solids) are listed in this appendix as maximum annual averages for the segment. Dissolved oxygen criteria are listed as minimum 24-hour means at any site within the segment. Absolute minima and seasonal criteria are listed in §307.7 of this title unless otherwise specified in this appendix.