Euphorbia Cyparissias
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Euphorbiaceae
Botanische Bestimmungsübungen 1 Euphorbiaceae Euphorbiaceae (Wolfsmilchgewächse) 1 Systematik und Verbreitung Die Euphorbiaceae gehören zu den Eudikotyledonen (Kerneudikotyledonen > Superrosiden > Rosiden > Fabiden). Innerhalb dieser wird die Familie zur Ordnung der Malpighiales (Malpighienartige) gestellt. Die Euphorbiaceae umfassen rund 230 Gattungen mit ca. 6.000 Arten. Sie werden in 4 Unterfamilien gegliedert: 1. Cheilosoideae, 2. Acalyphoideae, 3. Crotonoideae und 4. Euphorbioideae sowie in 6 Triben unterteilt. Die Familie ist überwiegend tropisch verbreitet mit einem Schwerpunkt im indomalaiischen Raum und in den neuweltlichen Tropen. Die Gattung Euphorbia (Wolfsmilch) ist auch in außertropischen Regionen wie z. B. dem Mittelmeerraum, in Südafrika sowie in den südlichen USA häufig. Heimisch ist die Familie mit Mercurialis (Bingelkraut; 2 Arten) und Euphorbia (Wolfsmilch; 20-30 Arten) vertreten. Abb. 1: Verbreitungskarte. 2 Morphologie 2.1 Habitus Die Familie ist sehr vielgestaltig. Es handelt sich um ein- und mehrjährige krautige Pflanzen, Halbsträucher, Sträucher bis große Bäume oder Sukkulenten. Besonders in S-Afrika und auf den Kanarischen Inseln kommen auf hitzebelasteten Trockenstandorten zahlreiche kakteenartige stammsukkulente Arten vor, die in den Sprossachsen immens viel Wasser speichern können. © PD DR. VEIT M. DÖRKEN, Universität Konstanz, FB Biologie Botanische Bestimmungsübungen 2 Euphorbiaceae Abb. 2: Lebensformen; entweder einjährige (annuelle) oder ausdauernde (perennierende) krautige Pflanzen, aber auch viele Halbsträucher, -

Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016
Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016 April 1981 Revised, May 1982 2nd revision, April 1983 3rd revision, December 1999 4th revision, May 2011 Prepared for U.S. Department of Commerce Ohio Department of Natural Resources National Oceanic and Atmospheric Administration Division of Wildlife Office of Ocean and Coastal Resource Management 2045 Morse Road, Bldg. G Estuarine Reserves Division Columbus, Ohio 1305 East West Highway 43229-6693 Silver Spring, MD 20910 This management plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Ohio Coastal Management Program. OWC NERR Management Plan, 2011 - 2016 Acknowledgements This management plan was prepared by the staff and Advisory Council of the Old Woman Creek National Estuarine Research Reserve (OWC NERR), in collaboration with the Ohio Department of Natural Resources-Division of Wildlife. Participants in the planning process included: Manager, Frank Lopez; Research Coordinator, Dr. David Klarer; Coastal Training Program Coordinator, Heather Elmer; Education Coordinator, Ann Keefe; Education Specialist Phoebe Van Zoest; and Office Assistant, Gloria Pasterak. Other Reserve staff including Dick Boyer and Marje Bernhardt contributed their expertise to numerous planning meetings. The Reserve is grateful for the input and recommendations provided by members of the Old Woman Creek NERR Advisory Council. The Reserve is appreciative of the review, guidance, and council of Division of Wildlife Executive Administrator Dave Scott and the mapping expertise of Keith Lott and the late Steve Barry. -

Integrated Noxious Weed Management Plan: US Air Force Academy and Farish Recreation Area, El Paso County, CO
Integrated Noxious Weed Management Plan US Air Force Academy and Farish Recreation Area August 2015 CNHP’s mission is to preserve the natural diversity of life by contributing the essential scientific foundation that leads to lasting conservation of Colorado's biological wealth. Colorado Natural Heritage Program Warner College of Natural Resources Colorado State University 1475 Campus Delivery Fort Collins, CO 80523 (970) 491-7331 Report Prepared for: United States Air Force Academy Department of Natural Resources Recommended Citation: Smith, P., S. S. Panjabi, and J. Handwerk. 2015. Integrated Noxious Weed Management Plan: US Air Force Academy and Farish Recreation Area, El Paso County, CO. Colorado Natural Heritage Program, Colorado State University, Fort Collins, Colorado. Front Cover: Documenting weeds at the US Air Force Academy. Photos courtesy of the Colorado Natural Heritage Program © Integrated Noxious Weed Management Plan US Air Force Academy and Farish Recreation Area El Paso County, CO Pam Smith, Susan Spackman Panjabi, and Jill Handwerk Colorado Natural Heritage Program Warner College of Natural Resources Colorado State University Fort Collins, Colorado 80523 August 2015 EXECUTIVE SUMMARY Various federal, state, and local laws, ordinances, orders, and policies require land managers to control noxious weeds. The purpose of this plan is to provide a guide to manage, in the most efficient and effective manner, the noxious weeds on the US Air Force Academy (Academy) and Farish Recreation Area (Farish) over the next 10 years (through 2025), in accordance with their respective integrated natural resources management plans. This plan pertains to the “natural” portions of the Academy and excludes highly developed areas, such as around buildings, recreation fields, and lawns. -

Fungal Pseudoflowers Can Influence the Fecundity of Insect-Pollinated Flowers on Euphorbia Cyparissias
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Bot. Helv. 116 (2006): 149 – 158 0253-1453/06/020149-10 DOI 10.1007/s00035-006-0762-y Birkhäuser Verlag, Basel, 2006 Botanica Helvetica Fungal pseudoflowers can influence the fecundity of insect-pollinated flowers on Euphorbia cyparissias Monika Pfunder1,2 and Bitty A. Roy3 1 Geobotanical Institute, ETH Zürich, Zürichbergstrasse 38, CH-8044 Zürich 2 Present address: Ecogenics GmbH, Wagistrasse 23, CH-8952 Schlieren e-mail: [email protected] 3 Center for Ecology and Evolutionary Biology, 5289 University of Oregon, Eugene, Oregon 97405-5289, USA Manuscript accepted 17 August 2006 Abstract Pfunder M. and Roy B.A. 2006. Fungal pseudoflowers can influence the fecundity of insect-pollinated flowers on Euphorbia cyparissias. Bot. Helv. 116: 149 – 158. Euphorbia cyparissias is often infected by a rust fungus from the species complex Uromyces pisi. Infected plants do not form flowers but pseudoflowers, rosettes of yellow leaves upon which the fungus presents gametes in a sweet-smelling sugary nectar. Insects feed on the nectar and transfer fungal gametes between mating types. Here we show that pseudoflowers and the flowers of non-infected hosts overlap in “flowering” for more than one month, even though pseudoflowers start “flowering” one month earlier than true flowers. As the fungus and its host also share insect visitors, we hypothesized that they might interact either by facilitating each others insect visits or by competing for “pollinators”. We addressed this question by weekly grid-mapping an Euphorbia population near Zermatt in the Swiss Alps and relating the average density and frequency around hosts and pseudoflowers during their “flowering” period to their fitness (success in seed set and spore production). -

Phylogeny of Rosids
Wind-pollinated Rosids Fabids Part I Announcements Lab Quiz today. Lecture review Tuesday, 3-4pm, HCK 320. Lecture Exam Wednesday. Arboretum Field Trip Wednesday. Phylogeny of angiosperms Angiosperms “Basal angiosperms” Parallel venation scattered vascular bundles 1 cotyledon Tricolpate pollen vessels (Jansen et al. 2007) Phylogeny of Eudicots (or Tricolpates) Eudicots (or Tricolpates) “Basal eudicots” (Soltis et al. 2011) Phylogeny of Rosids Rosids Saxifragales Saxifragaceae Crassulaceae Fabids: Malvids: Malpighiales Brassicales Salicaceae Brassicaceae Violaceae Malvales Euphorbiaceae Fabales Malvaceae Sapindales Fabaceae Rosales Aceraceae Myrtales Rosaceae Fagales Onagraceae Betulaceae Geraniales Fagaceae Geraniaceae (The Angiosperm Phylogeny Group 2009) Crassulaceae (Stonecrop family) http://www.blankees.com/house/plants/image/kalanchoe.jpg Kalanchoe sp. Echeveria derenbergii Echeveria sp. http://www.smgrowers.com/imagedb/Echeveria_derenbergii.JPG http://micheleroohani.com/blog/wp-content/uploads/2008/07/succulent-echeveria-michele-roohani-huntington.jpg Crassulacean Acid Metabolism http://hyperphysics.phy-astr.gsu.edu/hbase/biology/phoc.html#c4 Green Roofs http://en.wikipedia.org/wiki/Sedum Sedum acre biting stonecrop http://ecobrooklyn.com/extensive-green-roof/ Crassulaceae (Stonecrop family) 35 genera, 1500 species (Crassula, Echeveria, Kalanchoe, Sedum) Habit: Stem: Leaves: Tim Hagan 2006 Crassulaceae (Stonecrop family) Inflorescence: Flowers: Tim Hagan 2003 Sex of plant: Rod Gilbert 2006 Crassulaceae (Stonecrop family) Textbook -

Forest Health Technology Enterprise Team Biological Control of Invasive
Forest Health Technology Enterprise Team TECHNOLOGY TRANSFER Biological Control Biological Control of Invasive Plants in the Eastern United States Roy Van Driesche Bernd Blossey Mark Hoddle Suzanne Lyon Richard Reardon Forest Health Technology Enterprise Team—Morgantown, West Virginia United States Forest FHTET-2002-04 Department of Service August 2002 Agriculture BIOLOGICAL CONTROL OF INVASIVE PLANTS IN THE EASTERN UNITED STATES BIOLOGICAL CONTROL OF INVASIVE PLANTS IN THE EASTERN UNITED STATES Technical Coordinators Roy Van Driesche and Suzanne Lyon Department of Entomology, University of Massachusets, Amherst, MA Bernd Blossey Department of Natural Resources, Cornell University, Ithaca, NY Mark Hoddle Department of Entomology, University of California, Riverside, CA Richard Reardon Forest Health Technology Enterprise Team, USDA, Forest Service, Morgantown, WV USDA Forest Service Publication FHTET-2002-04 ACKNOWLEDGMENTS We thank the authors of the individual chap- We would also like to thank the U.S. Depart- ters for their expertise in reviewing and summariz- ment of Agriculture–Forest Service, Forest Health ing the literature and providing current information Technology Enterprise Team, Morgantown, West on biological control of the major invasive plants in Virginia, for providing funding for the preparation the Eastern United States. and printing of this publication. G. Keith Douce, David Moorhead, and Charles Additional copies of this publication can be or- Bargeron of the Bugwood Network, University of dered from the Bulletin Distribution Center, Uni- Georgia (Tifton, Ga.), managed and digitized the pho- versity of Massachusetts, Amherst, MA 01003, (413) tographs and illustrations used in this publication and 545-2717; or Mark Hoddle, Department of Entomol- produced the CD-ROM accompanying this book. -

Guidebook to Invasive Nonnative Plants of the Elwha Watershed Restoration
Guidebook to Invasive Nonnative Plants of the Elwha Watershed Restoration Olympic National Park, Washington Cynthia Lee Riskin A project submitted in partial fulfillment of the requirements for the degree of Master of Environmental Horticulture University of Washington 2013 Committee: Linda Chalker-Scott Kern Ewing Sarah Reichard Joshua Chenoweth Program Authorized to Offer Degree: School of Environmental and Forest Sciences Guidebook to Invasive Nonnative Plants of the Elwha Watershed Restoration Olympic National Park, Washington Cynthia Lee Riskin Master of Environmental Horticulture candidate School of Environmental and Forest Sciences University of Washington, Seattle September 3, 2013 Contents Figures ................................................................................................................................................................. ii Tables ................................................................................................................................................................. vi Acknowledgements ....................................................................................................................................... vii Introduction ....................................................................................................................................................... 1 Bromus tectorum L. (BROTEC) ..................................................................................................................... 19 Cirsium arvense (L.) Scop. (CIRARV) -

New Jersey Strategic Management Plan for Invasive Species
New Jersey Strategic Management Plan for Invasive Species The Recommendations of the New Jersey Invasive Species Council to Governor Jon S. Corzine Pursuant to New Jersey Executive Order #97 Vision Statement: “To reduce the impacts of invasive species on New Jersey’s biodiversity, natural resources, agricultural resources and human health through prevention, control and restoration, and to prevent new invasive species from becoming established.” Prepared by Michael Van Clef, Ph.D. Ecological Solutions LLC 9 Warren Lane Great Meadows, New Jersey 07838 908-637-8003 908-528-6674 [email protected] The first draft of this plan was produced by the author, under contract with the New Jersey Invasive Species Council, in February 2007. Two subsequent drafts were prepared by the author based on direction provided by the Council. The final plan was approved by the Council in August 2009 following revisions by staff of the Department of Environmental Protection. Cover Photos: Top row left: Gypsy Moth (Lymantria dispar); Photo by NJ Department of Agriculture Top row center: Multiflora Rose (Rosa multiflora); Photo by Leslie J. Mehrhoff, University of Connecticut, Bugwood.org Top row right: Japanese Honeysuckle (Lonicera japonica); Photo by Troy Evans, Eastern Kentucky University, Bugwood.org Middle row left: Mile-a-Minute (Polygonum perfoliatum); Photo by Jil M. Swearingen, USDI, National Park Service, Bugwood.org Middle row center: Canadian Thistle (Cirsium arvense); Photo by Steve Dewey, Utah State University, Bugwood.org Middle row right: Asian -

Euphorbia Esula
IPANE - Catalog of Species Search Results http://www.lib.uconn.edu/webapps/ipane/browsing.cfm?descriptionid=51 Home | Early Detection | IPANE Species | Data & Maps | Volunteers | About the Project | Related Information Catalog of Species Search Results Euphorbia esula (Leafy spurge ) :: Catalog of Species Search Common Name(s) | Full Scientific Name | Family Name Common | Family Scientific Name | Images | Synonyms | Description | Similar Species | Reproductive/Dispersal Mechanisms | Distribution | History of Introduction in New England | Habitats in New England | Threats | Early Warning Notes | Management Links | Documentation Needs | Additional Information | References | Data Retrieval | Maps of New England Plant Distribution COMMON NAME Leafy spurge FULL SCIENTIFIC NAME Euphorbia esula L. FAMILY NAME COMMON Spurge family FAMILY SCIENTIFIC NAME Euphorbiaceae IMAGES Incursion Inflorescence Habit close-up E. esula on right E. esula taller and E. plants and E. 1 of 8 9/24/2007 3:25 PM IPANE - Catalog of Species Search Results http://www.lib.uconn.edu/webapps/ipane/browsing.cfm?descriptionid=51 cyparissias on cyparissias left shorter plants in back NOMENCLATURE/SYNONYMS Synonyms: None DESCRIPTION Botanical Glossary Euphorbia esula is an herbaceous perennial that can reach a height of 30-70 cm (1-2.25 ft.). It is a colonial plant that has strong roots. The leaves and stems of the plant are bluish-green in color. The leaves are linear to lance-linear in shape and measure 3-8 cm (1.25-3 in.) long and 3-8 mm (0.1-0.3 in.) wide; they are alternate in their arrangement. The leaves that are just below the umbel are shorter and broader and are lanceolate to ovate in shape. -
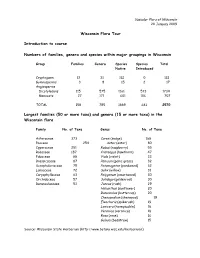
Wisconsin Flora Tour Introduction to Course Numbers of Families, Genera
Vascular Flora of Wisconsin 20 January 2009 Wisconsin Flora Tour Introduction to course Numbers of families, genera and species within major groupings in Wisconsin Group Families Genera Species Species Total Native Introduced Cryptogams 13 31 112 0 112 Gymnosperms 3 8 15 2 17 Angiosperms Dicotyledons 115 575 1161 573 1734 Monocots 27 171 601 106 707 TOTAL 158 785 1889 681 2570 Largest families (50 or more taxa) and genera (15 or more taxa) in the Wisconsin flora Family No. of Taxa Genus No. of Taxa Asteraceae 373 Carex (sedge) 168 Poaceae 254 Aster (aster) 80 Cyperaceae 251 Rubus (raspberry) 55 Rosaceae 187 Crateagus (hawthorn) 47 Fabaceae 88 Viola (violet) 33 Brassicaceae 87 Panicum (panic grass) 32 Scrophulariaceae 75 Potamogeton (pondweed) 32 Lamiaceae 72 Salix (willow) 31 Caryophyllaceae 63 Polygonum (smartweed) 30 Orchidaceae 57 Solidago (goldenrod) 30 Ranunculaceaee 53 Juncus (rush) 29 Helianthus (sunflower) 20 Ranunculus (buttercup) 20 Chenopodium (chenopod) 19 Eleocharis (spikerush) 19 Lonicera (honeysuckle) 18 Veronica (veronica) 18 Rosa (rose) 16 Galium (bedstraw) 15 Source: Wisconsin State Herbarium (http://www.botany.wisc.edu/herbarium/) Four major floristic elements in the Wisconsin flora Boreal Alleghenian Ozarkian Prairie Two floristic provinces Northern hardwood Prairie forests Tension Zone Brief look at four plant communities Beech maple or southern mesic Oak forest or southern xeric Prairie Bog or fen Vascular Flora of Wisconsin 22 January 2009 Nomenclature and Vascular Cryptogams I Nomenclature vs. Classification Rank -

Ecological Checklist of the Missouri Flora for Floristic Quality Assessment
Ladd, D. and J.R. Thomas. 2015. Ecological checklist of the Missouri flora for Floristic Quality Assessment. Phytoneuron 2015-12: 1–274. Published 12 February 2015. ISSN 2153 733X ECOLOGICAL CHECKLIST OF THE MISSOURI FLORA FOR FLORISTIC QUALITY ASSESSMENT DOUGLAS LADD The Nature Conservancy 2800 S. Brentwood Blvd. St. Louis, Missouri 63144 [email protected] JUSTIN R. THOMAS Institute of Botanical Training, LLC 111 County Road 3260 Salem, Missouri 65560 [email protected] ABSTRACT An annotated checklist of the 2,961 vascular taxa comprising the flora of Missouri is presented, with conservatism rankings for Floristic Quality Assessment. The list also provides standardized acronyms for each taxon and information on nativity, physiognomy, and wetness ratings. Annotated comments for selected taxa provide taxonomic, floristic, and ecological information, particularly for taxa not recognized in recent treatments of the Missouri flora. Synonymy crosswalks are provided for three references commonly used in Missouri. A discussion of the concept and application of Floristic Quality Assessment is presented. To accurately reflect ecological and taxonomic relationships, new combinations are validated for two distinct taxa, Dichanthelium ashei and D. werneri , and problems in application of infraspecific taxon names within Quercus shumardii are clarified. CONTENTS Introduction Species conservatism and floristic quality Application of Floristic Quality Assessment Checklist: Rationale and methods Nomenclature and taxonomic concepts Synonymy Acronyms Physiognomy, nativity, and wetness Summary of the Missouri flora Conclusion Annotated comments for checklist taxa Acknowledgements Literature Cited Ecological checklist of the Missouri flora Table 1. C values, physiognomy, and common names Table 2. Synonymy crosswalk Table 3. Wetness ratings and plant families INTRODUCTION This list was developed as part of a revised and expanded system for Floristic Quality Assessment (FQA) in Missouri. -

Field Checklist
14 September 2020 Cystopteridaceae (Bladder Ferns) __ Cystopteris bulbifera Bulblet Bladder Fern FIELD CHECKLIST OF VASCULAR PLANTS OF THE KOFFLER SCIENTIFIC __ Cystopteris fragilis Fragile Fern RESERVE AT JOKERS HILL __ Gymnocarpium dryopteris CoMMon Oak Fern King Township, Regional Municipality of York, Ontario (second edition) Aspleniaceae (Spleenworts) __ Asplenium platyneuron Ebony Spleenwort Tubba Babar, C. Sean Blaney, and Peter M. Kotanen* Onocleaceae (SensitiVe Ferns) 1Department of Ecology & Evolutionary Biology 2Atlantic Canada Conservation Data __ Matteuccia struthiopteris Ostrich Fern University of Toronto Mississauga Centre, P.O. Box 6416, Sackville NB, __ Onoclea sensibilis SensitiVe Fern 3359 Mississauga Road, Mississauga, ON Canada E4L 1G6 Canada L5L 1C6 Athyriaceae (Lady Ferns) __ Deparia acrostichoides SilVery Spleenwort *Correspondence author. e-mail: [email protected] Thelypteridaceae (Marsh Ferns) The first edition of this list Was compiled by C. Sean Blaney and Was published as an __ Parathelypteris noveboracensis New York Fern appendix to his M.Sc. thesis (Blaney C.S. 1999. Seed bank dynamics of native and exotic __ Phegopteris connectilis Northern Beech Fern plants in open uplands of southern Ontario. University of Toronto. __ Thelypteris palustris Marsh Fern https://tspace.library.utoronto.ca/handle/1807/14382/). It subsequently Was formatted for the web by P.M. Kotanen and made available on the Koffler Scientific Reserve Website Dryopteridaceae (Wood Ferns) (http://ksr.utoronto.ca/), Where it Was revised periodically to reflect additions and taxonomic __ Athyrium filix-femina CoMMon Lady Fern changes. This second edition represents a major revision reflecting recent phylogenetic __ Dryopteris ×boottii Boott's Wood Fern and nomenclatural changes and adding additional species; it will be updated periodically.