Understanding Habitat Selection of the Vulnerable Wild Yak Bos Mutus on the Tibetan Plateau
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bison Literature Review Biology
Bison Literature Review Ben Baldwin and Kody Menghini The purpose of this document is to compare the biology, ecology and basic behavior of cattle and bison for a management context. The literature related to bison is extensive and broad in scope covering the full continuum of domestication. The information incorporated in this review is focused on bison in more or less “wild” or free-ranging situations i.e.., not bison in close confinement or commercial production. While the scientific literature provides a solid basis for much of the basic biology and ecology, there is a wealth of information related to management implications and guidelines that is not captured. Much of the current information related to bison management, behavior (especially social organization) and practical knowledge is available through local experts, current research that has yet to be published, or popular literature. These sources, while harder to find and usually more localized in scope, provide crucial information pertaining to bison management. Biology Diet Composition Bison evolutional history provides the basis for many of the differences between bison and cattle. Bison due to their evolution in North America ecosystems are better adapted than introduced cattle, especially in grass dominated systems such as prairies. Many of these areas historically had relatively low quality forage. Bison are capable of more efficient digestion of low-quality forage then cattle (Peden et al. 1973; Plumb and Dodd 1993). Peden et al. (1973) also found that bison could consume greater quantities of low protein and poor quality forage then cattle. Bison and cattle have significant dietary overlap, but there are slight differences as well. -

Last Interglacial (MIS 5) Ungulate Assemblage from the Central Iberian Peninsula: the Camino Cave (Pinilla Del Valle, Madrid, Spain)
Palaeogeography, Palaeoclimatology, Palaeoecology 374 (2013) 327–337 Contents lists available at SciVerse ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Last Interglacial (MIS 5) ungulate assemblage from the Central Iberian Peninsula: The Camino Cave (Pinilla del Valle, Madrid, Spain) Diego J. Álvarez-Lao a,⁎, Juan L. Arsuaga b,c, Enrique Baquedano d, Alfredo Pérez-González e a Área de Paleontología, Departamento de Geología, Universidad de Oviedo, C/Jesús Arias de Velasco, s/n, 33005 Oviedo, Spain b Centro Mixto UCM-ISCIII de Evolución y Comportamiento Humanos, C/Sinesio Delgado, 4, 28029 Madrid, Spain c Departamento de Paleontología, Facultad de Ciencias Geológicas, Universidad Complutense de Madrid, Ciudad Universitaria, 28040 Madrid, Spain d Museo Arqueológico Regional de la Comunidad de Madrid, Plaza de las Bernardas, s/n, 28801-Alcalá de Henares, Madrid, Spain e Centro Nacional de Investigación sobre la Evolución Humana (CENIEH), Paseo Sierra de Atapuerca, s/n, 09002 Burgos, Spain article info abstract Article history: The fossil assemblage from the Camino Cave, corresponding to the late MIS 5, constitutes a key record to un- Received 2 November 2012 derstand the faunal composition of Central Iberia during the last Interglacial. Moreover, the largest Iberian Received in revised form 21 January 2013 fallow deer fossil population was recovered here. Other ungulate species present at this assemblage include Accepted 31 January 2013 red deer, roe deer, aurochs, chamois, wild boar, horse and steppe rhinoceros; carnivores and Neanderthals Available online 13 February 2013 are also present. The origin of the accumulation has been interpreted as a hyena den. Abundant fallow deer skeletal elements allowed to statistically compare the Camino Cave fossils with other Keywords: Early Late Pleistocene Pleistocene and Holocene European populations. -

Wild Yak Bos Mutus in Nepal: Rediscovery of a Flagship Species
Mammalia 2015; aop Raju Acharya, Yadav Ghimirey*, Geraldine Werhahn, Naresh Kusi, Bidhan Adhikary and Binod Kunwar Wild yak Bos mutus in Nepal: rediscovery of a flagship species DOI 10.1515/mammalia-2015-0066 2009). It has also been believed to inhabit the lower eleva- Received April 15, 2015; accepted July 21, 2015 tion Altai ranges in Mongolia (Olsen 1990). The possible fossil of wild yak discovered in Nepal (Olsen 1990) provided historical evidence of the species’ Abstract: Wild yak Bos mutus is believed to have gone presence in the country. Schaller and Liu (1996) also extinct from Nepal. Various searches in the last decade stated the occurrence of the wild yak in Nepal. Wild failed to document its presence. In Humla district, far- yak is said to inhabit the areas north of the Himalayas western Nepal, we used observation from transects and (Jerdon 1874, Hinton and Fry 1923), which also include vantage points, sign survey on trails, and informal dis- Limi Valley. Skins and horns were sporadically discov- cussions to ascertain the presence of wild yak in 2013 ered by explorers in the Himalayan and trans-Himala- (May–June) and 2014 (June–July). Direct sightings of two yan region of the country. These include evidence of individuals and hoof marks, dung piles, pelts, and head three horns of the species (around four decades old) of an individual killed in 2012 confirmed its presence. that can still be found in Lo Manthang (two pairs of The wild yak has an uncertain national status with con- horn) and Tsaile (one pair of horns) of Upper Mustang firmed records only from Humla district. -

Phylogeographical Analyses of Domestic and Wild Yaks
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Digital.CSIC RIGIN AL Phylogeographical analyses of domestic AR TI C L E and wild yaks based on mitochondrial DNA: new data and reappraisal Zhaofeng Wang1†, Xin Shen2†, Bin Liu2†, Jianping Su3†, Takahiro Yonezawa4, Yun Yu2, Songchang Guo3, Simon Y. W. Ho5, Carles Vila` 6, Masami Hasegawa4 and Jianquan Liu1* 1Molecular Ecology Group, Key Laboratory of AB STRACT Arid and Grassland Ecology, School of Life Science, Lanzhou University, Lanzhou 730000, Aim We aimed to examine the phylogeographical structure and demographic 2 history of domestic and wild yaks ( ) based on a wide range of Gansu, China, Beijing Genomics Institute, Bos grunniens Chinese Academy of Sciences, Beijing 101300, samples and complete mitochondrial genomic sequences. 3 China, Key Laboratory of Evolution and Location The Qinghai-Tibetan Plateau (QTP) of western China. Adaptation of Plateau Biota, Northwest Plateau Institute of Biology, Chinese Academy Methods All available D-loop sequences for 405 domesticated yaks and 47 wild of Sciences, Xining 810001, Qinghai, China, yaks were examined, including new sequences from 96 domestic and 34 wild yaks. 4School of Life Sciences, Fudan University, We further sequenced the complete mitochondrial genomes of 48 domesticated Shanghai 200433, China, 5Centre for and 21 wild yaks. Phylogeographical analyses were performed using the Macroevolution and Macroecology, Research mitochondrial D-loop and the total genome datasets. School of Biology, Australian National Results We recovered a total of 123 haplotypes based on the D-loop sequences University, Canberra, ACT 0200, Australia, in wild and domestic yaks. -

Structural Characterisation of Toll-Like Receptor 1 (TLR1) and Toll-Like
RVC OPEN ACCESS REPOSITORY – COPYRIGHT NOTICE This is the peer-reviewed, manuscript version of the following article: Woodman, S., Gibson, A. J., García, A. R., Contreras, G. S., Rossen, J. W., Werling, D. and Offord, V. (2016) 'Structural characterisation of Toll-like receptor 1 (TLR1) and Toll-like receptor 6 (TLR6) in elephant and harbor seals', Veterinary Immunology and Immunopathology, 169, 10-14. The final version is available online via http://dx.doi.org/10.1016/j.vetimm.2015.11.006. © 2016. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/. The full details of the published version of the article are as follows: TITLE: Structural characterisation of Toll-like receptor 1 (TLR1) and Toll-like receptor 6 (TLR6) in elephant and harbor seals AUTHORS: Woodman, S., Gibson, A. J., García, A. R., Contreras, G. S., Rossen, J. W., Werling, D. and Offord, V. JOURNAL TITLE: Veterinary Immunology and Immunopathology VOLUME: 169 PUBLISHER: Elsevier PUBLICATION DATE: January 2016 DOI: 10.1016/j.vetimm.2015.11.006 1 Structural characterisation of Toll-like receptor 1 (TLR1) and Toll-like receptor 6 (TLR6) in 2 elephant and harbor seals 3 4 Sally Woodman1,Amanda J. Gibson1, Ana Rubio García2, Guillermo Sanchez Contreras2, John W. 5 Rossen3, Dirk Werling1, Victoria Offord1,* 6 7 1Molecular Immunology Group, Department of Pathology and Pathogen Biology, Royal Veterinary 8 College, Hawkshead Lane, Hatfield, AL9 7TA, UK; 9 2Veterinary Department, Seal Rehabilitation and Research Centre (SRRC), Pieterburen, The 10 Netherlands; 11 3Department of Medical Microbiology, University of Groningen, University Medical Center 12 Groningen, Groningen, the Netherlands; 13 * Corresponding author at the Research Support Office, Royal Veterinary College, Hawkshead Lane, 14 Hatfield, AL9 7TA, UK Tel: ++44 1707 667038; E-Mail: [email protected] 15 16 1 1 Abstract 2 Pinnipeds are a diverse clade of semi-aquatic mammals, which act as key indicators of ecosystem health. -
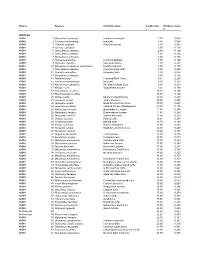
PDF File Containing Table of Lengths and Thicknesses of Turtle Shells And
Source Species Common name length (cm) thickness (cm) L t TURTLES AMNH 1 Sternotherus odoratus common musk turtle 2.30 0.089 AMNH 2 Clemmys muhlenbergi bug turtle 3.80 0.069 AMNH 3 Chersina angulata Angulate tortoise 3.90 0.050 AMNH 4 Testudo carbonera 6.97 0.130 AMNH 5 Sternotherus oderatus 6.99 0.160 AMNH 6 Sternotherus oderatus 7.00 0.165 AMNH 7 Sternotherus oderatus 7.00 0.165 AMNH 8 Homopus areolatus Common padloper 7.95 0.100 AMNH 9 Homopus signatus Speckled tortoise 7.98 0.231 AMNH 10 Kinosternon subrabum steinochneri Florida mud turtle 8.90 0.178 AMNH 11 Sternotherus oderatus Common musk turtle 8.98 0.290 AMNH 12 Chelydra serpentina Snapping turtle 8.98 0.076 AMNH 13 Sternotherus oderatus 9.00 0.168 AMNH 14 Hardella thurgi Crowned River Turtle 9.04 0.263 AMNH 15 Clemmys muhlenbergii Bog turtle 9.09 0.231 AMNH 16 Kinosternon subrubrum The Eastern Mud Turtle 9.10 0.253 AMNH 17 Kinixys crosa hinged-back tortoise 9.34 0.160 AMNH 18 Peamobates oculifers 10.17 0.140 AMNH 19 Peammobates oculifera 10.27 0.140 AMNH 20 Kinixys spekii Speke's hinged tortoise 10.30 0.201 AMNH 21 Terrapene ornata ornate box turtle 10.30 0.406 AMNH 22 Terrapene ornata North American box turtle 10.76 0.257 AMNH 23 Geochelone radiata radiated tortoise (Madagascar) 10.80 0.155 AMNH 24 Malaclemys terrapin diamondback terrapin 11.40 0.295 AMNH 25 Malaclemys terrapin Diamondback terrapin 11.58 0.264 AMNH 26 Terrapene carolina eastern box turtle 11.80 0.259 AMNH 27 Chrysemys picta Painted turtle 12.21 0.267 AMNH 28 Chrysemys picta painted turtle 12.70 0.168 AMNH 29 -

European Bison
IUCN/Species Survival Commission Status Survey and Conservation Action Plan The Species Survival Commission (SSC) is one of six volunteer commissions of IUCN – The World Conservation Union, a union of sovereign states, government agencies and non- governmental organisations. IUCN has three basic conservation objectives: to secure the conservation of nature, and especially of biological diversity, as an essential foundation for the future; to ensure that where the Earth’s natural resources are used this is done in a wise, European Bison equitable and sustainable way; and to guide the development of human communities towards ways of life that are both of good quality and in enduring harmony with other components of the biosphere. A volunteer network comprised of some 8,000 scientists, field researchers, government officials Edited by Zdzis³aw Pucek and conservation leaders from nearly every country of the world, the SSC membership is an Compiled by Zdzis³aw Pucek, Irina P. Belousova, unmatched source of information about biological diversity and its conservation. As such, SSC Ma³gorzata Krasiñska, Zbigniew A. Krasiñski and Wanda Olech members provide technical and scientific counsel for conservation projects throughout the world and serve as resources to governments, international conventions and conservation organisations. IUCN/SSC Action Plans assess the conservation status of species and their habitats, and specifies conservation priorities. The series is one of the world’s most authoritative sources of species conservation information -

Genetic Variation of Mitochondrial DNA Within Domestic Yak Populations J.F
Genetic variation of mitochondrial DNA within domestic yak populations J.F. Bailey,1 B. Healy,1 H. Jianlin,2 L. Sherchand,3 S.L. Pradhan,4 T. Tsendsuren,5 J.M. Foggin,6 C. Gaillard,7 D. Steane,8 I. Zakharov 9 and D.G. Bradley1 1. Department of Genetics, Trinity College, Dublin 2, Ireland 2. Department of Animal Science, Gansu Agricultural University, Lanzhou 730070, Gansu, P.R. China 3. Livestock Production Division, Department of Livestock Services, Harihar Bhawan, Pulchowk, Nepal 4. Resource Development Advisor, Nepal–Australia Community Resource Management Project, Kathmandu, Nepal 5. Institute of Biology, Academy of Sciences of Mongolia, Ulaan Baatar, Mongolia 6. Department of Biology, Arizona State University, Tempe, AZ 85287–1501 USA 7. Institute of Animal Breeding, University of Berne, Bremgarten-strasse 109a, CH-3012 Berne, Switzerland 8. FAO (Food and Agricultural Organization of the United Nations) Regional Office for Asia and the Pacific, 39 Phra Atit Road, Bangkok 10200, Thailand 9. Vavilov Institute of General Genetics Russian Academy of Sciences, Gubkin str., 3, 117809 GSP-1, Moscow B-333, Russia Summary Yak (Bos grunniens) are members of the Artiodactyla, family Bovidae, genus Bos. Wild yak are first observed at Pleistocene levels of the fossil record. We believed that they, together with the closely related species of Bos taurus, B. indicus and Bison bison, resulted from a rapid radiation of the genus towards the end of the Miocene. Today domestic yak live a fragile existence in a harsh environment. Their fitness for this environment is vital to their survival and to the millions of pastoralists who depend upon them. -

Phylogeny of Dictyocaulus (Lungworms) from Eight Species of Ruminants Based on Analyses of Ribosomal RNA Data
179 Phylogeny of Dictyocaulus (lungworms) from eight species of ruminants based on analyses of ribosomal RNA data J. HO¨ GLUND1*,D.A.MORRISON1, B. P. DIVINA1,2, E. WILHELMSSON1 and J. G. MATTSSON1 1 Department of Parasitology (SWEPAR), National Veterinary Institute and Swedish University of Agriculture Sciences, 751 89 Uppsala, Sweden 2 College of Veterinary Medicine, University of the Philippines Los Ban˜os, College, Laguna, 4031 Philippines (Received 8 November 2002; revised 8 February 2003; accepted 8 February 2003) SUMMARY In this study, we conducted phylogenetic analyses of nematode parasites within the genus Dictyocaulus (superfamily Trichostrongyloidea). Lungworms from cattle (Bos taurus), domestic sheep (Ovis aries), European fallow deer (Dama dama), moose (Alces alces), musk ox (Ovibos moschatus), red deer (Cervus elaphus), reindeer (Rangifer tarandus) and roe deer (Capreolus capreolus) were obtained and their small subunit ribosomal RNA (SSU) and internal transcribed spacer 2 (ITS2) sequences analysed. In the hosts examined we identified D. capreolus, D. eckerti, D. filaria and D. viviparus. However, in fallow deer we detected a taxon with unique SSU and ITS2 sequences. The phylogenetic position of this taxon based on the SSU sequences shows that it is a separate evolutionary lineage from the other recognized species of Dictyocaulus. Furthermore, the analysis of the ITS2 sequence data indicates that it is as genetically distinct as are the named species of Dictyocaulus. Therefore, either this taxon needs to be recognized as a new species, or D. capreolus, D. eckerti and D. viviparus need to be combined into a single species. Traditionally, the genus Dictyocaulus has been placed as a separate family within the superfamily Trichostrongyloidea. -

Mixed-Species Exhibits with Pigs (Suidae)
Mixed-species exhibits with Pigs (Suidae) Written by KRISZTIÁN SVÁBIK Team Leader, Toni’s Zoo, Rothenburg, Luzern, Switzerland Email: [email protected] 9th May 2021 Cover photo © Krisztián Svábik Mixed-species exhibits with Pigs (Suidae) 1 CONTENTS INTRODUCTION ........................................................................................................... 3 Use of space and enclosure furnishings ................................................................... 3 Feeding ..................................................................................................................... 3 Breeding ................................................................................................................... 4 Choice of species and individuals ............................................................................ 4 List of mixed-species exhibits involving Suids ........................................................ 5 LIST OF SPECIES COMBINATIONS – SUIDAE .......................................................... 6 Sulawesi Babirusa, Babyrousa celebensis ...............................................................7 Common Warthog, Phacochoerus africanus ......................................................... 8 Giant Forest Hog, Hylochoerus meinertzhageni ..................................................10 Bushpig, Potamochoerus larvatus ........................................................................ 11 Red River Hog, Potamochoerus porcus ............................................................... -

Idiograms of Yak (Bos Grunniens), Cattle (Bos Taurus) and Their Hybrid C.P
IDIOGRAMS OF YAK (BOS GRUNNIENS), CATTLE (BOS TAURUS) AND THEIR HYBRID C.P. Popescu To cite this version: C.P. Popescu. IDIOGRAMS OF YAK (BOS GRUNNIENS), CATTLE (BOS TAURUS) AND THEIR HYBRID. Annales de génétique et de sélection animale, INRA Editions, 1969, 1 (3), pp.207-217. hal- 00892361 HAL Id: hal-00892361 https://hal.archives-ouvertes.fr/hal-00892361 Submitted on 1 Jan 1969 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. IDIOGRAMS OF YAK (BOS GRUNNIENS), CATTLE (BOS TAURUS) AND THEIR HYBRID C.P. POPESCU Department of Genetics, Institute of Zootechnical Researches, 63, Dr Staicovici, Sector 6-Bucarest (Romania) (!‘) ABSTRACT The karyotypes of Yak (Bos grunniens L.) and Cattle (Bos taurus L.) are alike both numeri- cally and morphologically. However, idiograms of the two species reveal differences both in autosomes and in sexual chromosomes. The idiogram of hybrids (Bos grunniens L. S x Bos taurus L. !) represents roughly an average of parental idiograms. Numerous authors have studied the chromosomes of some hybrid animals and their parental species for the purpose of explaining the infertility of inter- specific hybrids (BASRUR and MooN, 1967; LAY and NADr,!R, ig6g; MA!NO et al., 1963). -

Holocene Distribution of European Bison – the Archaeozoological Record
MUNIBE (Antropologia-Arkeologia) 57 Homenaje a Jesús Altuna 421-428 SAN SEBASTIAN 2005 ISSN 1132-2217 The Holocene distribution of European bison – the archaeozoological record Distribución Holocena del bisonte europeo - el registro arqueozoológico KEY WORDS: Europe, Holocene, European bison, distribution, archaeozoological record. PALABRAS CLAVE: Europa, Holoceno, bisonte europeo, distribución, registro arqueozoológico. Norbert BENECKE* ABSTRACT The paper presents a reconstruction of the Holocene distribution of European bison or wisent. It is based on the archaeozoological record of this species. European bison was an early Postglacial immigrant into the European continent. The oldest evidence comes from sites in northern Central Europe and South Scandinavia dating to the Preboreal. In the Mid- and Late Holocene, European bison was widely distributed on the European continent. Its range extended from France in the west to the Ukraine and Russia in the east. Except for an area comprising East Poland, Belarus, Lithuania and Latvia, European bison was a rare species in most regions of its range. In the Middle Ages, there is a shrinkage of the range of wisent in its western part. RESUMEN El artículo presenta una reconstrucción de la distribución holocena del bisonte europeo. Está basada en el registro arqueozoológico de es- ta especie. El bisonte europeo fue un inmigrante al Continente europeo durante el Postglacial inicial. La más antigua evidencia procede de ya- cimientos del Norte de Europa Central y del Sur de Escandinavia, que datan del Preboreal. Durante el Holoceno medio y tardío el bisonte eu- ropeo estaba ampliamente distribuido en el Continente europeo. Su distribución se extendía desde Francia al W hasta Ucrania al E.