Anatomy of the Dromedary Head Skeleton Revisited
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anatomic Analysis of the Vidian Canal and the Surrounding
Braz J Otorhinolaryngol. 2019;85(2):136---143 Brazilian Journal of OTORHINOLARYNGOLOGY www.bjorl.org ORIGINAL ARTICLE The anatomic analysis of the vidian canal and the surrounding structures concerning vidian neurectomy ଝ using computed tomography scans a,∗ a b Gülay Ac¸ar , Aynur Emine C¸ic¸ekcibas¸ı , ˙Ibrahim C¸ukurova , c a d Kemal Emre Özen , Muzaffer ¸ekerS , ˙Ibrahim Güler a Necmettin Erbakan University, Meram Faculty of Medicine, Department of Anatomy, Konya, Turkey b Health Sciences University, Izmir Tepecik Trainig and Research Hospital, Department of Otolaryngology-Head and Neck Surgery, Izmir, Turkey c Katip C¸elebi University, Faculty of Medicine, Department of Anatomy, Izmir, Turkey d Selcuk University, Faculty of Medicine, Department of Radiology, Konya, Turkey Received 15 September 2017; accepted 8 November 2017 Available online 26 December 2017 KEYWORDS Abstract Intrasphenoid Introduction: The type of endoscopic approach chosen for vidian neurectomy can be specified septum; by evaluating the vidian canal and the surrounding sphenoid sinus structures. Morphometric Objective: The variations and morphometry of the vidian canal were investigated, focusing on analysis; the functional correlations between them which are crucial anatomical landmarks for preoper- Pterygoid process ative planning. pneumatization; Methods: This study was performed using paranasal multidetector computed tomography Vidian canal; images that were obtained with a section thickening of 0.625 mm of 250 adults. Vidian neurectomy Results: The distributions of 500 vidian canal variants were categorized as follows; Type 1, within the sphenoid corpus (55.6%); Type 2, partially protruding into the sphenoid sinus (34.8%); Type 3, within the sphenoid sinus (9.6%). The pneumatization of the pterygoid process is mostly seen in vidian canal Type 2 (72.4%) and Type 3 (95.8%) (p < 0.001). -

Anatomical Characteristics and Visibility of Mental Foramen and Accessory Mental Foramen: Panoramic Radiography Vs
Med Oral Patol Oral Cir Bucal. 2015 Nov 1;20 (6):e707-14. Radiographic study of the mental foramen variations Journal section: Oral Surgery doi:10.4317/medoral.20585 Publication Types: Research http://dx.doi.org/doi:10.4317/medoral.20585 Anatomical characteristics and visibility of mental foramen and accessory mental foramen: Panoramic radiography vs. cone beam CT Juan Muinelo-Lorenzo 1, Juan-Antonio Suárez-Quintanilla 2, Ana Fernández-Alonso 1, Jesús Varela-Mallou 3, María-Mercedes Suárez-Cunqueiro 4 1 PhD Student, Department of Stomatology, Medicine and Dentistry School, University of Santiago de Compostela, Spain 2 Associate Professor, Department of Anatomy, Medicine and Dentistry School, University of Santiago de Compostela, Spain 3 Professor and Chairman. Department of Social Psychology, Basic Psychology and Methodology, Psychology School, University of Santiago de Compostela, Spain 4 Associate Professor, Department of Stomatology, Medicine and Dentistry School, University of Santiago de Compostela, Spain Correspondence: Stomatology Department Medicine and Dentistry School University of Santiago de Compostela C/ Entrerrios S/N 15872 Muinelo-Lorenzo J, Suárez-Quintanilla JA, Fernández-Alonso A, Va- Santiago de Compostela, Spain rela-Mallou J, Suárez-Cunqueiro MM. Anatomical characteristics and [email protected] visibility of mental foramen and accessory mental foramen: Panoramic radiography vs. cone beam CT. Med Oral Patol Oral Cir Bucal. 2015 Nov 1;20 (6):e707-14. http://www.medicinaoral.com/medoralfree01/v20i6/medoralv20i6p707.pdf Received: 05/01/2015 Accepted: 05/05/2015 Article Number: 20585 http://www.medicinaoral.com/ © Medicina Oral S. L. C.I.F. B 96689336 - pISSN 1698-4447 - eISSN: 1698-6946 eMail: [email protected] Indexed in: Science Citation Index Expanded Journal Citation Reports Index Medicus, MEDLINE, PubMed Scopus, Embase and Emcare Indice Médico Español Abstract Background. -

Anatomy of Maxillary and Mandibular Local Anesthesia
Anatomy of Mandibular and Maxillary Local Anesthesia Patricia L. Blanton, Ph.D., D.D.S. Professor Emeritus, Department of Anatomy, Baylor College of Dentistry – TAMUS and Private Practice in Periodontics Dallas, Texas Anatomy of Mandibular and Maxillary Local Anesthesia I. Introduction A. The anatomical basis of local anesthesia 1. Infiltration anesthesia 2. Block or trunk anesthesia II. Review of the Trigeminal Nerve (Cranial n. V) – the major sensory nerve of the head A. Ophthalmic Division 1. Course a. Superior orbital fissure – root of orbit – supraorbital foramen 2. Branches – sensory B. Maxillary Division 1. Course a. Foramen rotundum – pterygopalatine fossa – inferior orbital fissure – floor of orbit – infraorbital 2. Branches - sensory a. Zygomatic nerve b. Pterygopalatine nerves [nasal (nasopalatine), orbital, palatal (greater and lesser palatine), pharyngeal] c. Posterior superior alveolar nerves d. Infraorbital nerve (middle superior alveolar nerve, anterior superior nerve) C. Mandibular Division 1. Course a. Foramen ovale – infratemporal fossa – mandibular foramen, Canal -> mental foramen 2. Branches a. Sensory (1) Long buccal nerve (2) Lingual nerve (3) Inferior alveolar nerve -> mental nerve (4) Auriculotemporal nerve b. Motor (1) Pterygoid nerves (2) Temporal nerves (3) Masseteric nerves (4) Nerve to tensor tympani (5) Nerve to tensor veli palatine (6) Nerve to mylohyoid (7) Nerve to anterior belly of digastric c. Both motor and sensory (1) Mylohyoid nerve III. Usual Routes of innervation A. Maxilla 1. Teeth a. Molars – Posterior superior alveolar nerve b. Premolars – Middle superior alveolar nerve c. Incisors and cuspids – Anterior superior alveolar nerve 2. Gingiva a. Facial/buccal – Superior alveolar nerves b. Palatal – Anterior – Nasopalatine nerve; Posterior – Greater palatine nerves B. -

Yahaya Et Al.Cdr
Anatomical Study of the Variations of the Facial bones in Skull of the Camel (Camelus dromendarius) in Nigeria SUMMARY wide opening on each side of the nasal The morphological features of facial bone and could be classified into two region of the camel skull were types (fIssura frontomaxillaris and investigated. A total of 42 camel skulls fissura nasofrontomaxillaris) (30 mature and 12 immature) from according to various patterns of three geographical locations articulations of the neighbouring (Maiduguri, Kano and Sokoto) in bones. The nasal region Nigeria were used in this study. The morphological information provided morphological features of the nasal in this study will contribute to region of the camel skulls were knowledge of the morphological observed to be composed of the os pattern of the fissures of facial bones in nasale, os incisivivum, os lacrimale, skull that can play a prominent role in maxilla and part of the os frontalale as osteological investigation or reported for other domestic animals. osteoarchaeology, and also offer Variations in the morphological elements for eventual comparative arrangement of the incisive, maxilla studies that can be used for tracing and nasal bones of the nasal region in origin of the animal. camel skulls for both mature and Key words: Morphology, variations, immature animals were observed to facial bones, skull, camel show two typical variations as nasomaxilloincisive notch (80%) and nasoincisive notch (20%) in all samples studied. Slit-like fissures were also observed at the frontomaxillary suture area in 90.5% mature and 92% immature camel skulls. These fissures were observed to be either rectangular or oval in shape and bilateral in 95% while in the remaining were either absent or unilateral. -

Morphology and Fracture Effects of the Hamulus Pterygoid: a Literature Review of the Last 49 Years
Latin American Journal of Development, Curitiba, v. 3, n. 1, p. 475-487, jan./feb. 2021. ISSN 2674-9297 Morphology and fracture effects of the hamulus pterygoid: a literature review of the last 49 years Morfología y efectos de fractura del hamulus pterygoid: una revisión de la literatura de los últimos 49 años DOI: 10.46814/lajdv3n1-041 Recebimento dos originais: 30/10/2020 Aceitação para publicação: 23/12/2020 Polyanne Junqueira Silva Andresen Strini PhD, Federal University of Uberlândia - UFU, Uberlândia, MG, Brazil Address: Rio Preto Street, 178, Lídice, Uberlândia - MG Paulinne Junqueira Silva Andresen Strini PhD, Federal University of Uberlândia - UFU, Uberlândia, MG, Brazil Address: Rio Preto Street, 178, Lídice, Uberlândia - MG ABSTRACT The hamulus pterygoid consists in a relevant anatomical structure, important for fixation of several tendons and muscles, keeping the integrity of soft palate and pharynx. A literature review was conducted in order to investigate the morphology and effect of hamulus pterygoid fracture in clinical manifestation and its relationships with other orofacial components. A literature search was conducted, using Pubmed and Bireme data bases, and covering the time period 1970 to 2019. The Key words for the research were hamulus pterygoid, pterygoid fracture and hamulus pterygoid fracture, resulting in 440 articles, being 41 initials selected. Among them, just 31 were included in the analysis and 08 of the articles were not available through our library system or were in volumes before our holdings began. The remaining were excluded when they weren’t in English idiom, or when didn’t talk about morphological, functional or damages in the hamulus pterygoid. -

Anatomy of the Dog the Present Volume of Anatomy of the Dog Is Based on the 8Th Edition of the Highly Successful German Text-Atlas of Canine Anatomy
Klaus-Dieter Budras · Patrick H. McCarthy · Wolfgang Fricke · Renate Richter Anatomy of the Dog The present volume of Anatomy of the Dog is based on the 8th edition of the highly successful German text-atlas of canine anatomy. Anatomy of the Dog – Fully illustrated with color line diagrams, including unique three-dimensional cross-sectional anatomy, together with radiographs and ultrasound scans – Includes topographic and surface anatomy – Tabular appendices of relational and functional anatomy “A region with which I was very familiar from a surgical standpoint thus became more comprehensible. […] Showing the clinical rele- vance of anatomy in such a way is a powerful tool for stimulating students’ interest. […] In addition to putting anatomical structures into clinical perspective, the text provides a brief but effective guide to dissection.” vet vet The Veterinary Record “The present book-atlas offers the students clear illustrative mate- rial and at the same time an abbreviated textbook for anatomical study and for clinical coordinated study of applied anatomy. Therefore, it provides students with an excellent working know- ledge and understanding of the anatomy of the dog. Beyond this the illustrated text will help in reviewing and in the preparation for examinations. For the practising veterinarians, the book-atlas remains a current quick source of reference for anatomical infor- mation on the dog at the preclinical, diagnostic, clinical and surgical levels.” Acta Veterinaria Hungarica with Aaron Horowitz and Rolf Berg Budras (ed.) Budras ISBN 978-3-89993-018-4 9 783899 9301 84 Fifth, revised edition Klaus-Dieter Budras · Patrick H. McCarthy · Wolfgang Fricke · Renate Richter Anatomy of the Dog The present volume of Anatomy of the Dog is based on the 8th edition of the highly successful German text-atlas of canine anatomy. -

Inferior Alveolar Nerve Trajectory, Mental Foramen Location and Incidence of Mental Nerve Anterior Loop
Med Oral Patol Oral Cir Bucal. 2017 Sep 1;22 (5):e630-5. CBCT anatomy of the inferior alveolar nerve Journal section: Oral Surgery doi:10.4317/medoral.21905 Publication Types: Research http://dx.doi.org/doi:10.4317/medoral.21905 Inferior alveolar nerve trajectory, mental foramen location and incidence of mental nerve anterior loop Miguel Velasco-Torres 1, Miguel Padial-Molina 1, Gustavo Avila-Ortiz 2, Raúl García-Delgado 3, Andrés Ca- tena 4, Pablo Galindo-Moreno 1 1 DDS, PhD, Department of Oral Surgery and Implant Dentistry, School of Dentistry, University of Granada, Granada, Spain 2 DDS, MS, PhD, Department of Periodontics, College of Dentistry, University of Iowa, Iowa City, USA 3 Specialist in Dental and Maxillofacial Radiology. Private Practice. Granada, Spain 4 PhD, Department of Experimental Psychology, School of Psychology, University of Granada, Granada, Spain Correspondence: School of Dentistry, University of Granada 18071 - Granada, Spain [email protected] Velasco-Torres M, Padial-Molina M, Avila-Ortiz G, García-Delgado R, Catena A, Galindo-Moreno P. Inferior alveolar nerve trajectory, mental foramen location and incidence of mental nerve anterior loop. Med Oral Received: 07/03/2017 Accepted: 21/06/2017 Patol Oral Cir Bucal. 2017 Sep 1;22 (5):e630-5. http://www.medicinaoral.com/medoralfree01/v22i5/medoralv22i5p630.pdf Article Number: 21905 http://www.medicinaoral.com/ © Medicina Oral S. L. C.I.F. B 96689336 - pISSN 1698-4447 - eISSN: 1698-6946 eMail: [email protected] Indexed in: Science Citation Index Expanded Journal Citation Reports Index Medicus, MEDLINE, PubMed Scopus, Embase and Emcare Indice Médico Español Abstract Background: Injury of the inferior alveolar nerve (IAN) is a serious intraoperative complication that may occur during routine surgical procedures, such as dental implant placement or extraction of impacted teeth. -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -
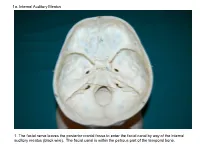
1A. Internal Auditory Meatus
1a. Internal Auditory Meatus 1. The facial nerve leaves the posterior cranial fossa to enter the facial canal by way of the internal auditory meatus (black wire). The facial canal is within the petrous part of the temporal bone. 1b. Internal Auditory Meatus The facial nerve leaves the posterior cranial fossa to enter the facial canal by way of the internal auditory meatus (black wire). 2. Hiatus of the Canal and Groove for the Greater Superficial Petrosal Nerve The greater superficial petrosal nerve leaves the facial canal to enter the middle cranial fossa by way of the hiatus of the canal for the greater superficial petrosal nerve (black wire). 3. Pterygoid Canal at Anterior Lip of the Lacerate Foramen The greater superficial petrosal nerve is joined by the deep petrosal nerve to form the nerve of the pterygoid canal (black and red wire). This nerve leaves the middle cranial fossa to enter the pterygopalatine fossa by way of the pterygoid canal. The posterior opening of the pterygoid canal is at the anterior lip of the lacerate foramen. The greater superficial nerve and the deep petrosal nerve travel within the cavernous sinus. 4. Pterygopalatine Fossa Seen Through the Pterygomaxillary Fissure The anterior opening of the pterygoid canal is into the pterygopalatine fossa (black wire). The pterygopalatine fossa is located medial to the pterygomaxillary fissure and contains the pterygopalatine ganglion. 5. External Auditory Meatus The chorda tympani nerve leaves the facial canal and crosses the middle ear (black wire). It then leaves the middle ear to arrive in the infratemporal fossa by way of the petrotympanic fissure. -

Chapter 2 Implants and Oral Anatomy
Chapter 2 Implants and oral anatomy Associate Professor of Maxillofacial Anatomy Section, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University Tatsuo Terashima In recent years, the development of new materials and improvements in the operative methods used for implants have led to remarkable progress in the field of dental surgery. These methods have been applied widely in clinical practice. The development of computerized medical imaging technologies such as X-ray computed tomography have allowed detailed 3D-analysis of medical conditions, resulting in a dramatic improvement in the success rates of operative intervention. For treatment with a dental implant to be successful, it is however critical to have full knowledge and understanding of the fundamental anatomical structures of the oral and maxillofacial regions. In addition, it is necessary to understand variations in the topographic and anatomical structures among individuals, with age, and with pathological conditions. This chapter will discuss the basic structure of the oral cavity in relation to implant treatment. I. Osteology of the oral area The oral cavity is composed of the maxilla that is in contact with the cranial bone, palatine bone, the mobile mandible, and the hyoid bone. The maxilla and the palatine bones articulate with the cranial bone. The mandible articulates with the temporal bone through the temporomandibular joint (TMJ). The hyoid bone is suspended from the cranium and the mandible by the suprahyoid and infrahyoid muscles. The formation of the basis of the oral cavity by these bones and the associated muscles makes it possible for the oral cavity to perform its various functions. -

A Description of the Geological Context, Discrete Traits, and Linear Morphometrics of the Middle Pleistocene Hominin from Dali, Shaanxi Province, China
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 150:141–157 (2013) A Description of the Geological Context, Discrete Traits, and Linear Morphometrics of the Middle Pleistocene Hominin from Dali, Shaanxi Province, China Xinzhi Wu1 and Sheela Athreya2* 1Laboratory for Human Evolution, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, China 2Department of Anthropology, Texas A&M University, College Station, TX 77843 KEY WORDS Homo heidelbergensis; Homo erectus; Asia ABSTRACT In 1978, a nearly complete hominin Afro/European Middle Pleistocene Homo and align it fossil cranium was recovered from loess deposits at the with Asian H. erectus.Atthesametime,itdisplaysa site of Dali in Shaanxi Province, northwestern China. more derived morphology of the supraorbital torus and It was subsequently briefly described in both English supratoral sulcus and a thinner tympanic plate than and Chinese publications. Here we present a compre- H. erectus, a relatively long upper (lambda-inion) occi- hensive univariate and nonmetric description of the pital plane with a clear separation of inion and opis- specimen and provide comparisons with key Middle thocranion, and an absolute and relative increase in Pleistocene Homo erectus and non-erectus hominins brain size, all of which align it with African and Euro- from Eurasia and Africa. In both respects we find pean Middle Pleistocene Homo. Finally, traits such as affinities with Chinese H. erectus as well as African the form of the frontal keel and the relatively short, and European Middle Pleistocene hominins typically broad midface align Dali specifically with other referred to as Homo heidelbergensis.Specifically,the Chinese specimens from the Middle Pleistocene Dali specimen possesses a low cranial height, relatively and Late Pleistocene, including H. -

Introduction to the Skeletal System and the Axial Skeleton 155
chapter Introduction to the 7 Skeletal System and the Axial Skeleton CHAPTER OVERVIEW OBJECTIVES 7.1 Introduction to the Skeletal System ……………… 153 1. Describe the gross anatomy and structure of a long 7.2 Bone Structure ………………………………………… 154 bone 7.3 Bone Histology ………………………………………… 155 2. Describe and compare the underlying histology of spongy and compact bone. 7.4 The Human Skeleton: Axial and Appendicular Divisions …………………………………………………… 156 3. List the five general shapes of bones. 7.5 Bone Classification and Markings ………………… 157 4. Describe and compare the different kinds of bone markings visible on the skeleton. 7.6 Axial Skeleton …………………………………………… 159 7.6a Cranium 5. Identify the components of the axial skeleton: cranial, 7.6b Facial facial, hyoid, vertebra, ribs and sternum. 7.6c Hyoid Bone 7.6d Vertebral Column 7.6e Thoracic Cage 7.1 Introduction to the Skeletal System The skeletal system serves to support the body’s soft tissues and to protect the body’s soft internal organs. Another important function that the bones have is to store materials such as calcium, phosphorus and lipids. Additionally, blood cells are synthesized in the red bone marrow to be released into the bloodstream. Bones serve as levers for the muscular system, working with them to produce movement and maintain posture. The human body contains 2 major kinds of bone tissue: compact and spongy. Compact bone (dense bone) is found on the outer surface of bones and serves as a place to absorb most of the stress on the bones. Spongy bone (cancellous tissue) is found on the inside of the compact bone layer.