SCULPTRA® Aesthetic (Injectable Poly-L-Lactic Acid)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
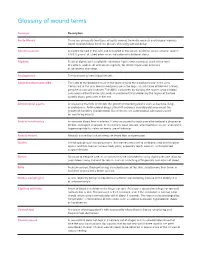
Wounds Definitions
Glossary of wound terms Acronym Description Acute Wound There are principally two types of acute wound; traumatic wounds and surgical wounds. Acute wounds follow the three phases of healing without delay. Albumin (serum) A protein formed in the liver and circulated in the serum. A normal serum albumin level is 3.5-5.0 grams/ dl. Used often as an indication of nutritional status. Alginate A salt of alginic acid, a colloidal substance from brown seaweed; used, in the form of calcium, sodium, or ammonium alginate, for dental impression materials or absorptive dressings. Angiogenesis The formation of new blood vessels. Ankle-Brachial Index (ABI) The ratio of the blood pressure in the lower legs to the blood pressure in the arms. Compared to the arm, lower blood pressure in the leg is an indication of blocked arteries (peripheral vascular disease). The ABI is calculated by dividing the higher systolic blood pressure in either the dorsalis pedis or posterior tibial arteries by the higher of the two systolic blood pressures in the arm. Antimicrobial agents A substance that kills or inhibits the growth of microorganisms such as bacteria, fungi, or protozoans. Antimicrobial drugs either kill microbes (microbicidal) or prevent the growth of microbes (microbistatic). Disinfectants are antimicrobial substances used on non-living objects. Arterial insufficiency Inadequate blood flow in arteries. It may be caused by occlusive atherosclerotic plaques or emboli; damaged, diseased, or intrinsically weak vessels; arteriovenous fistulas; aneurysms; hypercoagulability states; or heavy use of tobacco. Arterial wound Wounds caused by lack of adequate blood flow and perfusion. Biofilm A thick grouping of microorganisms that are very resistant to antibiotics and antimicrobial agents and that lives on various body parts, especially teeth, wounds, and implanted surgical devices. -

In the United States Court of Federal Claims OFFICE of SPECIAL MASTERS No
In the United States Court of Federal Claims OFFICE OF SPECIAL MASTERS No. 17-325V Filed: December 28, 2020 * * * * * * * * * * * * * * * * * * * * * * * * * * * * SHARON LABOUNTY, * * TO BE PUBLISHED Petitioner, * * * v. * Special Master Katherine E. Oler * SECRETARY OF HEALTH AND * HUMAN SERVICES, * * Chronic Regional Pain Syndrome * (CRPS); Flu Vaccine; Needle Stick Respondent. * * * * * * * * * * * * * * * * * * * * * * * * * * * * Howard Gold, Gold Law Firm, LLC, Wellesley Hills, MA for Petitioner Christine Becer, U.S. Department of Justice, Washington, DC, for Respondent RULING ON ENTITLEMENT1 On March 9, 2017, Sharon LaBounty (“Ms. LaBounty” or “Petitioner”) filed a petition pursuant to the National Vaccine Injury Compensation Program, 42 U.S.C. § 300aa-10.2 (“Vaccine Act” or “the Program”) alleging that the flu vaccination she received on September 18, 2015 caused her to suffer a reaction which was diagnosed as brachial plexopathy and brachial neuritis. Petition at 1, ECF No. 1. Petitioner filed an amended petition (“Amended Pet. 1”) on August 15, 2018 alleging her flu vaccination caused her to develop a shoulder injury related to vaccine administration (“SIRVA”). Amended Pet. 1 at 1, ECF No. 23. On September 15, 2018, Petitioner filed a second amended petition (“Amended Pet. 2”) alleging that the flu vaccination caused her to develop Chronic Regional Pain Syndrome (“CRPS”). Amended Pet. 2 at 1, ECF No. 24. 1 This Ruling will be posted on the United States Court of Federal Claims’ website, in accordance with the E-Government Act of 2002, 44 U.S.C. § 3501 (2012). This means the Ruling will be available to anyone with access to the internet. As provided in 42 U.S.C. § 300aa-12(d)(4)(B), however, the parties may object to the Ruling’s inclusion of certain kinds of confidential information. -

(IHS) the International Classification of Headache Disorders
ICHD-3 Cephalalgia 2018, Vol. 38(1) 1–211 ! International Headache Society 2018 Reprints and permissions: sagepub.co.uk/journalsPermissions.nav DOI: 10.1177/0333102417738202 journals.sagepub.com/home/cep Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition Copyright Translations The 3rd edition of the International Classification of Headache Disorders (ICHD-3) may be reproduced The International Headache Society (IHS) expressly freely for scientific, educational or clinical uses by insti- permits translations of all or parts of ICHD-3 for the tutions, societies or individuals. Otherwise, copyright purposes of clinical application, education, field testing belongs exclusively to the International Headache or other research. It is a condition of this permission Society. Reproduction of any part or parts in any that all translations are registered with IHS. Before manner for commercial uses requires the Society’s per- embarking upon translation, prospective translators mission, which will be granted on payment of a fee. are advised to enquire whether a translation exists Please contact the publisher at the address below. already in the proposed language. ßInternational Headache Society 2013–2018. All translators should be aware of the need to Applications for copyright permissions should be sub- use rigorous translation protocols. Publications report- mitted to Sage Publications Ltd, 1 Oliver’s Yard, 55 ing studies making use of translations of all or any part City Road, London EC1Y 1SP, United Kingdom of ICHD-3 should include a brief description of the (tel: þ44 (0) 207 324 8500; fax: þ44 (0) 207 324 8600; translation process, including the identities of the trans- [email protected]) (www.uk.sagepub.com). -

Pancreatitis in Cats
Getting Nutrition into a Cat Craig Webb, DVM, DACVIM Colorado State University Fort Collins, CO Our understanding of feline nutrition has advanced significantly from the day when we simply considered them small dogs, and the number of options we now have for dietary intervention in this species has expanded exponentially. But neither the knowledge of feline metabolism nor the number of available diets helps us, or the cat, one bit, if we can’t get the stuff into them. When a Labrador retriever refuses to eat we know the prognosis is grave: when a cat refuses to eat it may well be that they have decided that the presentation of their latest meal was not up to standards. Unlike Labrador retrievers, cats are one-trial learners, so make the mistake of trying sneak a medication into the one particular flavor of food the cat will tolerate, and that may well be the last time you get anything into that cat’s mouth. Try to switch diets on a Labrador and you might get a brief pause as the dog considers the phrase “fool me once, please!”. Try to switch diets on a cat, for its own good mind you, and suffer an expression of disdain and an attitude of incredulous indignation. So of course, what is perhaps the single most common clinical expression of almost anything wrong with a cat? A decreased-to-absent appetite. And what are the consequences of anorexia in a cat compared to a Labrador? Well cats have their own specific condition for just that – hepatic lipidosis. -

Special Considerations: 6 Neonatology: 1
Special Considerations: 6 Neonatology: 1 UNIT TERMINAL OBJECTIVE 6-1.1 At the completion of this unit, the paramedic student will be able to integrate pathophysiological principles and assessment findings to formulate a field impression and implement a treatment plan for a neonatal patient. COGNITIVE OBJECTIVES At the completion of this unit, the paramedic student will be able to: 6-1.2 Define the term newborn.(C-1) 6-1.3 Define the term neonate. (C-1) 6-1.4 Identify important antepartum factors that can affect childbirth. (C-1) 6-1.5 Identify important intrapartum factors that can term the newborn high risk. (C-1) 6-1.6 Identify the factors that lead to premature birth and low birth weight newborns. (C-1) 6-1.7 Distinguish between primary and secondary apnea. (C-3) 6-1.8 Discuss pulmonary perfusion and asphyxia. (C-1) 6-1.9 Identify the primary signs utilized for evaluating a newborn during resuscitation. (C-1) 6-1.10 Formulate an appropriate treatment plan for providing initial care to a newborn. (C-3) 6-1.11 Identify the appropriate use of the APGAR score in caring for a newborn.(C-1) 6-1.12 Calculate the APGAR score given various newborn situations. (C-3) 6-1.13 Determine when ventilatory assistance is appropriate for a newborn. (C-1) 6-1.14 Prepare appropriate ventilation equipment, adjuncts and technique for a newborn. (C-1) 6-1.15 Determine when chest compressions are appropriate for a newborn. (C-1) 6-1.16 Discuss appropriate chest compression techniques for a newborn. -

The Interface Reaction Pattern in the Skin: an Integrated Review of Clinical and Pathological Features☆,☆☆ Maria A
Human Pathology (2019) 91,86–113 www.elsevier.com/locate/humpath Current topics The interface reaction pattern in the skin: an integrated review of clinical and pathological features☆,☆☆ Maria A. Deschaine MD a, Julia S. Lehman MD a,b,⁎ aDepartment of Dermatology, Mayo Clinic, Rochester, MN 55905 bDepartment of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN 55905 Received 9 May 2019; revised 18 June 2019; accepted 20 June 2019 Keywords: Summary Not uncommonly, pathologists encounter biopsies displaying inflammation at the dermoepi- Interface; dermal junction and confronted with its numerous diagnostic possibilities. As with other inflammatory Lichenoid; dermatoses, the correct diagnosis relies on careful integration of clinical, laboratory, and histopathologi- Vacuolar; cal features. Knowledge of clinical aspects of these disorders is crucial, and at times, lack of training Inflammatory dermatoses in clinical dermatology can make clinicopathological correlation challenging for the pathologist. This re- view is organized following the classical classification of cell-poor (vacuolar) and cell-rich (lichenoid) interface processes. The various entities are described based on their clinical presentation along their clinical differential diagnosis followed by their histopathological features and pathological differential diagnosis. Our aim is to provide an updated, clinically relevant review that integrates nuanced clinical and pathological features, with an emphasis on clues that may help differentiate entities in the differential -
Basic Principles of Dermatology 0 Whitney A
OVERVIEW OF BASIC SCIENCE SECTION 1 Basic Principles of Dermatology 0 Whitney A. High, Carlo Francesco Tomasini, Giuseppe Argenziano and Iris Zalaudek Chapter Contents Etiologic Premises All students of dermatology, whether beginners or advanced scholars, Introduction to clinical dermatology . 1 require a basic conceptual framework upon which to organize thou- The role of dermatopathology in clinicopathologic correlation . 11 sands of skin diseases. A useful arrangement is one that is analogous to a tree, with a trunk, major branches, minor branches, twigs and, Introduction to the use of dermoscopy (dermatoscopy) . 32 ultimately, leaves (Fig. 0.1). Instead of memorizing thousands of leaves, a logical, progressive movement along the limbs will allow for a more complete and sophisticated differential diagnosis. Inflammatory versus neoplastic INTRODUCTION TO CLINICAL DERMATOLOGY An early and major “branch point” in classifying skin diseases is decid- The skin represents the largest organ of the human body. The average ing simply if a skin condition is “neoplastic” (either benign or malig- adult has 1.75 m2 (18.5 ft2) of skin that contains a variety of complex nant) or “inflammatory” (either infectious or non-infectious) (see Fig. adnexal structures, including hair follicles, nails, glands and special- 0.1). However, an experienced clinician knows that one must consider ized sensory structures, all of which function in protection, homeo- possible diagnoses along multiple limbs before narrowing the differen- stasis, and the transmission of sensation. Dermatology is the field tial diagnosis, because both overlap and mimicry can occur. For example, of medicine that deals with the macroscopic study of skin, adjacent mycosis fungoides, the most common form of cutaneous T-cell lym- mucosa (oral and genital) and cutaneous adnexa, while dermatopa- phoma, is a clonal lymphoproliferative disorder (a “neoplasm”), yet its thology deals with the microscopic study of the same structures. -

Clinical Guidelines
Clinical guidelines Exported on 11/23/2019 Clinical guidelines Clinical guidelines - Diagnosis and treatment manual For curative programmes in hospitals and dispensaries Guidance for prescribing © Médecins Sans Frontières, 2019 All rights reserved for all countries. No reproduction, translation and adaptation may be done without the prior permission of the Copyright owner. Médecins Sans Frontières. Clinical guidelines - Diagnosis and treatment manual. 2019 edition. ISBN 978-2-37585-041-1 Clinical guidelines - Diagnosis and treatment manual – 2 Clinical guidelines Table of contents • Authors/Contributors(see page 6) • Preface(see page 7) • Abbreviations and acronyms(see page 8) • Chapter 1: A few symptoms and syndromes(see page 10) • Shock(see page 11) • Seizures(see page 17) • Hypoglycaemia(see page 21) • Fever(see page 23) • Pain(see page 26) • Anaemia(see page 33) • Severe acute malnutrition(see page 38) • Chapter 2: Respiratory diseases(see page 43) • Acute upper airway obstruction(see page 44) • Rhinitis and rhinopharyngitis (common cold)(see page 47) • Acute sinusitis(see page 48) • Acute pharyngitis(see page 50) • Diphtheria(see page 53) • Other upper respiratory tract infections(see page 56) • Otitis(see page 60) • Whooping cough (pertussis)(see page 63) • Bronchitis(see page 65) • Bronchiolitis(see page 67) • Acute pneumonia(see page 69) • Staphylococcal pneumonia(see page 75) • Asthma(see page 77) • Pulmonary tuberculosis(see page 82) • Chapter 3: Gastrointestinal disorders(see page 84) • Acute diarrhoea(see page 85) • Shigellosis(see -

Fever with Rash Learning Objectives
FEVER WITH RASH LEARNING OBJECTIVES Review high yield causes of fever with rash Review the clinical presentation of Meningococcemia, Kawasaki Disease, GAS, and a few viral exams Review management of the aforementioned clinical scenarios GROUP A STREPTOCOCCUS Aka Streptococcus pyogenes Two major virulence factors Hyaluronic acid capsule prevents phagocytosis M Protein, a surface protein that prevents opsonization and and phagocytosis, facilitates tissue invasion Streptococcal toxins; superantigens; stimulate massive cytokine release Noninvasive infections Strep pharyngitis can lead to Acute Rheumatic Fever or PSGN Scarlett fever Impetigo Erysipelas and cellulitis can lead to PSGN Invasive infections Streptococcal Toxic Shock Syndrome Acute Necrotizing Fasciitis SCARLET FEVER Caused by exotoxin commonly associated with pharyngitis, but can be due to skin infections (pyrogenic exotoxin/erythrogenic exotoxin) Less frequent in areas where antibiotics are commonly used Rash begins on upper chest/neck 1-2 days after the onset of infection and spreads to trunk and extremities Diffuse erythema that blanches sandpaper texture of skin Pinpoint areas of deeper red scattered petechiae w/o blanching Pastia’s lines Other findings include circumoral pallor, strawberry tongue, eventual desquamation (after 3-4 days) Treat the underlying infection PCN/amoxicillin, cephalexin, clarithromycin or clindamycin Sandpaper Rash Pastia’s Lines Strawberry Tongue KAWASAKI DISEASE Acute febrile illness of childhood characterized by vasculitis -

December 2006
jucmcov_1206 11/29/06 10:23 AM Page 1 ™ JUCM ™ THE JOURNAL OF URGENT CARE MEDICINE www.jucm.com | The Official Publication of the Urgent Care Association of America DECEMBER 2006 VOLUME 1, NUMBER 2 FEATURES 10 Urticaria and Angioedema: A Case- Based Discussion 29 A Delicate Balance: Managing Your Practice, Caring for Your Patients DEPARTMENTS 16 Abstracts in Urgent Care 20 Insights in Images 34 Health Law 35 Occupational Medicine 38 Coding Q&A 40 Developing Data PUBLICATION BRAVEHEART A Project1 11/28/06 3:51 PM Page 1 Coming soon. From the makers of PATANOL® solution (olopatadine hydrochloride ophthalmic solution) 0.2% ©2006 Alcon, Inc. 10/06 PAT06511JA 38480_CHC-OL200006_CS_Dec_ASz_r1 1 11/7/06 5:54:35 PM editor_1206 11/29/06 10:42 AM Page 1 LETTER FROM THE EDITOR-IN-CHIEF The Power of Research edical progress is driven by Ultimately, these research topics, and many others like research. Opinions must be con- them, will raise urgent care medicine where it belongs—to tinuously challenged in order the forefront of the healthcare delivery discussion. Mto assure the greatest likelihood I encourage all of our readers to transform the undeniable of efficacy, quality, and safety. Over enthusiasm and passion you have for the value and virtues the last century, research has focused of urgent care medicine into an equivalent passion for prov- on examining the innovative diag- ing its value and virtues. There are many research resources nostic and therapeutic approaches to available through your local medical schools, hospitals, and disease and prevention that form the medical societies, as well as grant money available through backbone of clinical medicine as we know it today. -

Phenomena in Dermatology Article
Review Phenomena in dermatology Article Bhushan Madke, Bhavana Doshi, Sushil Pande1, Uday Khopkar Department of Dermatology, ABSTRACT Seth GS Medical College and KEM Hospital, Parel, Mumbai For a better understanding of various dermatoses, it is imperative for any physician practising - 400 012, 1Department of Dermatology, NKP Salve dermatology to have a good theoretical knowledge of the underlying pathophysiologic Institute of Medical Sciences processes involved in various systemic diseases involving the skin. For an easy grasp over and Lata Mangeshkar this topic, we have discussed the various phenomena under three broad categories, like (a) Hospital, Hingna, Nagpur, India clinical – Meyerson, Meirowsky, pathergy, Renbok, (b) laboratory – LE cell, prozone and (c) histopathology – Splendore-Hoeppli. Address for correspondence: Dr. Uday Khopkar, Department of Dermatology, Key words: Borst Jadassohn, Olfleck’s, Splendore Hoeppli, phenomenon, dermatology Seth GS Medical College and KEM Hospital, Mumbai - 400 012, India. E-mail: [email protected] “To study the phenomenon of disease without books is Phenomena in dermato-venereology can be divided to sail an uncharted sea, while to study books without into three broad categories, those described in a clinical patients is not to go to sea at all.” setting, in the laboratory or as a histopathological observation [Table 1]. Sir William Osler- British (Canadian born) physician (1849–1919) CLINICAL PHENOMENA INTRODUCTION Koebner/Isomorphic phenomenon In 1877, Heinrich Koebner described the appearance Phenomenon is defined as any sign or objective of psoriatic lesions in the uninvolved skin of symptom; any observable occurrence or fact.[1] In the psoriatic patients as a consequence of trauma.[2] This dermatological literature, the term phenomenon has phenomenon has been dealt in greater detail by several been applied to several peculiar clinical occurrences or laboratory observations. -

Appendix A: Table of Primary Lesions and Related Disorders
Appendix A: Table of Primary Lesions and Related Disorders Bullae Erysipelas [Part III] Erythema multiforme [Part III] Fixed drug eruption [Part III] Impetigo [Part VI] Tinea (large, multiloculated) [Part III] Urticaria (bullae as secondary lesions) [Part III] Macules Actinic keratosis (erythematous) [Part V] Atypical nevi [Part V] Common benign nevi (pigmented) [Part V] Ephelides [Part V] Erysipelas (erythematous) [Part III] Erythema multiforme (erythematous) [Part III] Erythrasma [Part III] Fixed drug eruption [Part III] Halo nevi [Part V] Impetigo (deep red) [Part VI] Lentigines [Part V] Malignant melanoma [Part V] Nodules Acne [Part VI] Basal cell carcinoma (translucent, dome-shaped) [Part V] Keratoacanthoma (dome-shaped) [Part V] Malignant melanoma [Part V] Molluscum (dome-shaped umbilicated) [Part II] Rosacea (red) [Part VI] Squamous cell carcinoma (indurated) [Part V] Verruca vulgaris [Part II] Papules Acne (with or without comedones) [Part VI] Atopic dermatitis [Part IV] Atypical nevi [Part V] Basal cell carcinoma (translucent, dome-shaped) [Part V] Developed dermal nevi (sharply defined) [Part V] DLE (sharply defined, raised, smooth, shiny) [Part IV] Early compound nevi (dome-shaped) [Part V] Erythema multiforme (erythematous) [Part III] From: Current Clinical Practice: Dermatology Skills for Primary Care: An Illustrated Guide D.J. Trozak, D.J. Tennenhouse, and J.J. Russell © Humana Press, Totowa, NJ 369 370 Appendices Halo nevi [Part V] Keratoacanthoma (dome-shaped) [Part V] Lichen planus (flat-topped, angular, polygonal) [Part