STIBNITE-QUARTZ DEPOSITS (MODELS 27D,E and 36C; Bliss and Orris, 1986A-C; Berger, 1993)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stibnite Gold Project
STIBNITE GOLD PROJECT PAYETTE & BOISE NATIONAL FORESTS INTERMOUNTAIN REGION August 22, 2018 STIBNITE MINING DISTRICT, PAYETTE NATIONAL FOREST, KRASSEL RANGER DISTRICT Regarding Locatable Minerals , 36 CFR 228, Subpart A: requires the Forest Service to: "Where conflicting interests Respond to a mining plan, evaluate that plan must be reconciled, the Consider requirements to minimize adverse effects to the extent feasible question shall always be Comply with applicable laws, regulations and answered from the stand- standards for environmental protection point of the greatest good of Assure appropriate reclamation Further respond by following National Environmental the greatest number in the Policy Act (NEPA) processes. long run." - James Wilson, Secretary Per 36 CFR 228.4, the Forest Service is required to take of Agriculture, 1905 in a letter to Gifford action to ensure that “operations are conducted so as, Pinchot, 1st Chief of the Forest Service. where feasible, to minimize adverse environmental impacts on National Forest surface resources. 1 Table of Contents Project Context STIBNITE GOLD Project Location 4 Mining History at Stibnite 5 PROJECT Who is Midas Gold 6 Stibnite Plan of Operations PAYETTE & BOISE NATIONAL Proposed Mining Plan of FOREST - INTERMOUNTAIN REGION Operations 7 Open Pit Mining 8 Anadromous Fish - Tunnel 9 Inventoried Roadless Areas - Why is this project of interest to many Access and Powerline 10 people and organizations? Facilities During Mining Operations 10 5 million recoverable ounces of gold x -

The Krásno Sn-W Ore District Near Horní Slavkov: Mining History, Geological and Mineralogical Characteristics
Journal of the Czech Geological Society 51/12(2006) 3 The Krásno Sn-W ore district near Horní Slavkov: mining history, geological and mineralogical characteristics Sn-W rudní revír Krásno u Horního Slavkova historie tìby, geologická a mineralogická charakteristika (47 figs, 1 tab) PAVEL BERAN1 JIØÍ SEJKORA2 1 Regional Museum Sokolov, Zámecká 1, Sokolov, CZ-356 00, Czech Republic 2 Department of Mineralogy and Petrology, National Museum, Václavské nám. 68, Prague 1, CZ-115 79, Czech Republic The tin-tungsten Krásno ore district near Horní Slavkov (Slavkovský les area, western Bohemia) belongs to the most important areas of ancient mining in the Czech Republic. The exceptionally rich and variable mineral associations, and the high number of mineral species, make this area one of the most remarkable mineralogical localities on a worldwide scale. The present paper reviews the data on geological setting of the ore district, individual ore deposits and mining history. Horní Slavkov and Krásno were known as a rich source of exquisite quality mineral specimens stored in numerous museum collections throughout Europe. The old museum specimens are often known under the German locality names of Schlaggenwald (= Horní Slavkov) and Schönfeld (=Krásno). The megascopic properties and paragenetic position of selected mineral classics are reviewed which include arsenopyrite, fluorapatite, fluorite, hübnerite, chalcopyrite, carpholite, cassiterite, quartz, molybdenite, rhodochrosite, sphalerite, topaz and scheelite. Key words: Sn-W ores; tin-tungsten mineralization; mining history; ore geology; mineralogy; Slavkovský les; Krásno, Horní Slavkov ore district; Czech Republic. Introduction valleys dissected parts of the area. In the ore district area, the detailed surface morphology is modified by large de- In the mining history of Central Europe, Bohemia and pressions caused by the collapse of old underground Moravia are known as important source of gold, silver, workings and by extensive dumps. -

40 Common Minerals and Their Uses
40 Common Minerals and Their Uses Aluminum Beryllium The most abundant metal element in Earth’s Used in the nuclear industry and to crust. Aluminum originates as an oxide called make light, very strong alloys used in the alumina. Bauxite ore is the main source aircraft industry. Beryllium salts are used of aluminum and must be imported from in fluorescent lamps, in X-ray tubes and as Jamaica, Guinea, Brazil, Guyana, etc. Used a deoxidizer in bronze metallurgy. Beryl is in transportation (automobiles), packaging, the gem stones emerald and aquamarine. It building/construction, electrical, machinery is used in computers, telecommunication and other uses. The U.S. was 100 percent products, aerospace and defense import reliant for its aluminum in 2012. applications, appliances and automotive and consumer electronics. Also used in medical Antimony equipment. The U.S. was 10 percent import A native element; antimony metal is reliant in 2012. extracted from stibnite ore and other minerals. Used as a hardening alloy for Chromite lead, especially storage batteries and cable The U.S. consumes about 6 percent of world sheaths; also used in bearing metal, type chromite ore production in various forms metal, solder, collapsible tubes and foil, sheet of imported materials, such as chromite ore, and pipes and semiconductor technology. chromite chemicals, chromium ferroalloys, Antimony is used as a flame retardant, in chromium metal and stainless steel. Used fireworks, and in antimony salts are used in as an alloy and in stainless and heat resisting the rubber, chemical and textile industries, steel products. Used in chemical and as well as medicine and glassmaking. -

The Mineralogical Fate of Arsenic During Weathering Of
THE MINERALOGICAL FATE OF ARSENIC DURING WEATHERING OF SULFIDES IN GOLD-QUARTZ VEINS: A MICROBEAM ANALYTICAL STUDY A Thesis Presented to the faculty of the Department of Geology California State University, Sacramento Submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in Geology by Tamsen Leigh Burlak SPRING 2012 © 2012 Tamsen Leigh Burlak ALL RIGHTS RESERVED ii THE MINERALOGICAL FATE OF ARSENIC DURING WEATHERING OF SULFIDES IN GOLD-QUARTZ VEINS: A MICROBEAM ANALYTICAL STUDY A Thesis by Tamsen Leigh Burlak Approved by: __________________________________, Committee Chair Dr. Charles Alpers __________________________________, Second Reader Dr. Lisa Hammersley __________________________________, Third Reader Dr. Dave Evans ____________________________ Date iii Student: Tamsen Leigh Burlak I certify that this student has met the requirements for format contained in the University format manual, and that this thesis is suitable for shelving in the Library and credit is to be awarded for the project. _______________________, Graduate Coordinator ___________________ Dr. Dave Evans Date Department of Geology iv Abstract of THE MINERALOGICAL FATE OF ARSENIC DURING WEATHERING OF SULFIDES IN GOLD-QUARTZ VEINS: A MICROBEAM ANALYTICAL STUDY by Tamsen Leigh Burlak Mine waste piles within the historic gold mining site, Empire Mine State Historic Park (EMSHP) in Grass Valley, California, contain various amounts of arsenic and are the current subject of remedial investigations to characterize the arsenic present. In this study, electron microprobe, QEMSCAN (Quantitative Evaluation of Minerals by SCANning electron microscopy), and X-ray absorption spectroscopy (XAS) were used collectively to locate and identify the mineralogical composition of primary and secondary arsenic-bearing minerals at EMSHP. -

Controls on Antimony Speciation and Mobility in Legacy Mine Tailings Environments: a Case Study of Mineral Occurrences in the Tintina Gold Province, Alaska and Yukon
Controls on Antimony Speciation and Mobility in Legacy Mine Tailings Environments: A Case Study of Mineral Occurrences in the Tintina Gold Province, Alaska and Yukon. USGS Award MRERP 06HQGR0177 (Principle Investigator T.P. Trainor) T.P. Trainor1, S.H. Mueller2,*, V. Ritchie1, R.J. Goldfarb2 1.University of Alaska Fairbanks, Dept. of Chemistry and Biochemistry, P.O. Box 756160, Fairbanks, AK 99775-6160, Phone: 907-474-5628, Email: [email protected] 2. U.S. Geological Survey, Mineral Resources Program, Denver, CO 80225 * Present Address: Water Management Consultants Inc., 3845 North Business Center Drive, Tucson, AZ 85705, Phone: 520-319-0725 Research supported by the U.S. Geological Survey (USGS), Department of the Interior, under USGS award number 06HQGR0177. The views and conclusions contained in this document are those of the author(s) and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the U.S. Government. Introduction In recent years, a great deal of progress has been made in the development of geoenvironmental models to predict the potential for environmental contamination associated with mineral resource development (c.f. du Bray, 1995; Plumlee and Logsdon, 1999). The application of such models to current, prospective, and legacy mining sites provides industry, public land managers, and environmental quality agencies an important tool for developing management and remediation strategies. An essential aspect of developing such models is access to high quality water, sediment, and soil chemistry data sets from well-characterized mineral deposits. These data sets can be used to predict the identity and levels of potentially toxic trace elements based on similarities in ore deposit mineralogy, host rock lithology, and other geo-environmental variables. -

Clinocervantite, ~-Sb204, the Natural Monoclinic Polymorph of Cervantite from the Cetine Mine, Siena, Italy
Eur. J. Mineral. 1999,11,95-100 Clinocervantite, ~-Sb204, the natural monoclinic polymorph of cervantite from the Cetine mine, Siena, Italy RICCARDO BASSO 1, GABRIELLA LUCCHETTI I, LIVIa ZEFIRO 1 and ANDREA PALENZONA 2 IDipartimento di Scienze delia Terra dell'Universita, Corso Europa 26, 1-]6] 32 Genova, Ita]y e-mail: minera]@dister.unige.it 2Dipartimento di Chi mica e Chimica industria]e dell'Universita, Via Dodecaneso 3],1-]6]46 Genova, Ita]y Abstract: Clinocervantite occurs at the Cetine di Cotorniano mine associated with valentinite, tripuhyite, bindheimite and rosiaite. Clinocervantite, appearing generally as aggregates of single prisms elongated along [001] or twinned on {100}, is co]ourless, transparent, with vitreous lustre, biaxial, with the lowest measured ] ] refractive index a,' = .72 and the highest one y' = 2. O. The strongest lines in the powder pattern are dill = 3.244 A and d311 = 2.877 A. The crystal structure, space group C2Ie with a = 12.061(1) A, b = 4.836(1) A, ] e = 5.383( I) A, ~= 04.60( 4)" and Z = 4, has been refined to R = 0.020, confirming the new mineral to be the natural analogue of the synthetic ~-Sb204 already known. The structures of clinocervantite and cervantite may be regarded as built up by stacking layers of nearly identical structure and composition accounting for both polytypism in the Sb204 compound and twinning of the clinocervantite crystals. Key-words: clinocervantite, crystal-structure refinement, cervantite, twinning. Introduction Occurrence, physical properties and chemical composition During the study of rosiaite (Basso et al., 1996), an associated new mineral was found in materia] Clinocervantite occurs in litt]e cavities of a rock from the Cetine mine, central Tuscany, Italy. -

The Forming Conditions of Alyaskitovoe Tin-Tungsten Deposit, Russia
0393-000066 The forming conditions of Alyaskitovoe tin-tungsten deposit, Russia Corresponding author: Elena Anikina, IGEM RAS, [email protected] Co-authors: Gennady Gamyanin, IGEM RAS, [email protected] The Alyaskitovoe Sn-W deposit is located at the boundary between the Kular-Nersko terrane and the Verkhoyansk fold-thrust belt. It is localized in the tin-tungsten sublatitudinal metallogenic zone. The deposit area is composed of Upper Triassic sandstone-shale intruded by granite porphyry stock (98 MA Rb-Sr). Stock refers to the formation of young Li-F granites (P2O5 = 0.22- 0.56%, F = 0.12-0.21%, Sr = 9224-688g/t, Li = 47-252g/t, Rb = 61-194g/t). Stock and enclosing hornfels dissected by series of steeply dipping quartz veins meridional strike (L = up to 700 m, M = 0.4-0.6 m). Veins are accompanied by wallrock changes. It is greisenization in granitoids (up to 3 m) and tourmalinization – in hornfels (0.5 m). Several paragenetic associations are identified. Metasomatic: molybdenite-apatite1-tourmaline1- quartz-muscovite, tourmaline2-apatite2-fluorite-arsenopyrite-hydromica. Ore: cassiterite- wolframite-tourmaline-arsenopyrite-quartz, pyrrhotite-stannite-sphalerite-pyrite, molybdenite- matildite-aramayoite-galena-(Bi-andorite) Sb-gustovite, hubnerite-Ag-sulfoantimonite-calcite- quartz. Fluid inclusions (FI) in quartz of greisen and ore veins were studied. Temperature of homogenization of greisen inclusions is 460-480°C. FI contain weakly concentrated solutions (4.9- 3.3 wt.% NaCl-equiv), the gas phase is presented СО2 (29.6-78,2 mol.%), СН4 (11.1-21.8 mol.%). FI in quartz of ore veins contain a liquid phase with concentration 9.2-3.3 wt.% NaCl-equiv and homogenization temperatures of 290-380°С. -

Schafarzikite from the Type Locality Pernek (Malé Karpaty Mountains, Slovak Republic) Revisited
Eur. J. Mineral. 2007, 19, 419–427 Schafarzikite from the type locality Pernek (Malé Karpaty Mountains, Slovak Republic) revisited Jˇ´ SEJKORA1,*, D OZDÍN2,Jˇ´ VITÁLOŠ3,P TUCEKˇ 4,Jˇ´ CEJKAˇ 1 and R DUˇ DAˇ 5 1 Department of Mineralogy and Petrology, National Museum, Václavské nám. 68, 115 79, Praha 1, Czech Republic *Corresponding author, e-mail: [email protected] 2 Department of Mineralogy and Petrology, Faculty of Natural Sciences, Comenius University, Mlynská dolina, 84215 Bratislava, Slovak Republic 3 Šenkvická cesta 9, 90201 Pezinok, Slovak Republic 4 Klíž 80, 95845 Vel’ký Klíž, Slovak Republic 5 Eastern Slovak Museum, Hviezdoslavova 3, 04136 Košice, Slovak Republic Abstract: A rare mineral schafarzikite, an oxide of Fe2+ and Sb3+, was found after more than 80 years at the type locality near Pernek (Malé Karpaty Mountains, Slovak Republic). Crystals, druses, and crusts of schafarzikite occur on fractures in quartz- carbonate-stibnite hydrothermal ores. The Sb mineralization is bound to black shales and phyllites in a zone of actinolitic rocks. Associated minerals include ankerite, berthierite, stibnite, valentinite, kermesite, senarmontite, and gypsum. Prismatic crystals of schafarzikite are 0.1–1.0 mm, rarely up to 1.5 mm large, with the dominant forms {110}, {121}, {112}, {010}, {221}, {131}, and {231}. The optical properties are: uniaxial, with relatively strong pleochroism in red-brown tints; refraction indices higher than 1.74; the average refraction index, calculated from the Gladstone-Dale equation, is 2.001. The physical properties of schafarzikite from Pernek are: dark brown to black color, adamantine to metallic luster, brown streak; translucent (brown to orange) in very thin fragments; good {100} cleavage and perfect cleavage along unindexed planes parallel to z axis, tenacity-brittle; VHN10g micro- hardness = 251 and 278 kp/mm2 (for two cuts with differing orientation), corresponding to Mohs’ hardness of 3.5–4; calculated 3 density Dx = 5.507 g/cm . -

Tungsten Minerals and Deposits
DEPARTMENT OF THE INTERIOR FRANKLIN K. LANE, Secretary UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, Director Bulletin 652 4"^ TUNGSTEN MINERALS AND DEPOSITS BY FRANK L. HESS WASHINGTON GOVERNMENT PRINTING OFFICE 1917 ADDITIONAL COPIES OF THIS PUBLICATION MAY BE PROCURED FROM THE SUPERINTENDENT OF DOCUMENTS GOVERNMENT PRINTING OFFICE WASHINGTON, D. C. AT 25 CENTS PER COPY CONTENTS. Page. Introduction.............................................................. , 7 Inquiries concerning tungsten......................................... 7 Survey publications on tungsten........................................ 7 Scope of this report.................................................... 9 Technical terms...................................................... 9 Tungsten................................................................. H Characteristics and properties........................................... n Uses................................................................. 15 Forms in which tungsten is found...................................... 18 Tungsten minerals........................................................ 19 Chemical and physical features......................................... 19 The wolframites...................................................... 21 Composition...................................................... 21 Ferberite......................................................... 22 Physical features.............................................. 22 Minerals of similar appearance................................. -

Clarke Jeff a 201709 Mscproj
THE CHARACTERIZATION OF ARSENIC MINERAL PHASES FROM LEGACY MINE WASTE AND SOIL NEAR COBALT, ONTARIO by Jeff Clarke A research project submitted to the Department of Geological Sciences and Geological Engineering In conformity with the requirements for the degree of Master of Science in Applied Geology Queen’s University Kingston, Ontario, Canada (July, 2017) Copyright © Jeff Clarke, 2017 i ABSTRACT The Cobalt-Coleman silver (Ag) mining camp has a long history of mining dating back to 1903. Silver mineralization is hosted within carbonate veins and occurs in association with Fe-Co-Ni arsenide and sulpharsenide mineral species. The complex mineralogy presented challenges to early mineral processing methods with varying success of Ag recovery and a significant amount of arsenic (As) in waste material which was disposed in the numerous tailings deposits scattered throughout the mining camp, and in many instances disposed of uncontained. The oxidation and dissolution of As-bearing mineral phases in these tailings and legacy waste sites releases As into the local aquatic environment. Determining the distribution of primary and secondary As mineral species in different legacy mine waste materials provides an understanding of the stability of As. Few studies have included detailed advanced mineralogical characterization of As mineral species from legacy mine waste in the Cobalt area. As part of this study, a total of 28 samples were collected from tailings, processed material near mill sites and soils from the legacy Nipissing and Cart Lake mining sites. The samples were analyzed for bulk chemistry to delineate material with strongly elevated As returned from all sample sites. This sampling returned highly elevated As with up to 6.01% As from samples near mill sites, 1.71% As from tailings and 0.10% As from soils. -
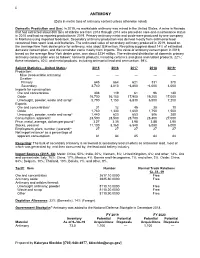
Antimony Data Sheet
22 ANTIMONY (Data in metric tons of antimony content unless otherwise noted) Domestic Production and Use: In 2019, no marketable antimony was mined in the United States. A mine in Nevada that had extracted about 800 tons of stibnite ore from 2013 through 2014 was placed on care-and-maintenance status in 2015 and had no reported production in 2019. Primary antimony metal and oxide were produced by one company in Montana using imported feedstock. Secondary antimony production was derived mostly from antimonial lead recovered from spent lead-acid batteries. The estimated value of secondary antimony produced in 2019, based on the average New York dealer price for antimony, was about $34 million. Recycling supplied about 14% of estimated domestic consumption, and the remainder came mostly from imports. The value of antimony consumption in 2019, based on the average New York dealer price, was about $234 million. The estimated distribution of domestic primary antimony consumption was as follows: nonmetal products, including ceramics and glass and rubber products, 22%; flame retardants, 40%; and metal products, including antimonial lead and ammunition, 39%. Salient Statistics—United States: 2015 2016 2017 2018 2019e Production: Mine (recoverable antimony) — — — — — Smelter: Primary 645 664 621 331 370 Secondary 3,740 3,810 e3,800 e4,000 4,000 Imports for consumption: Ore and concentrates 308 119 61 96 140 Oxide 16,700 16,100 17,900 19,200 17,000 Unwrought, powder, waste and scrap1 5,790 7,150 6,830 6,500 7,200 Exports: Ore and concentrates1 31 -

C:\Documents and Settings\Alan Smithee\My Documents\MOTM
Itmd1//8Lhmdq`knesgdLnmsg9Rshamhsd This month’s mineral is among the most collectible of all minerals. Ours come from a Chinese antimony mine that is now recognized as a classic stibnite locality, and our write-up explains the unusual structure and properties of stibnite, describes its remarkable crystals, and details the many uses of antimony. OVERVIEW PHYSICAL PROPERTIES Chemistry: Sb2S3 Antimony Trisulfide, often containing some bismuth Class: Sulfides Group: Stibnite Crystal System: Orthorhombic Crystal Habits: Individual bladed or acicular prisms or jumbled aggregates of prisms, striated lengthwise; prisms often bent or twisted; also as radiated groups and granular and massive forms. Color: Lead-gray and gray-black to steel-gray and silvery-gray, sometimes with a bluish hue; occasionally iridescent; tarnish black. Luster: Metallic Transparency: Opaque Streak: Dark lead-gray Refractive Index: None (opaque) Cleavage: Perfect in one direction lengthwise Figure 1. Stibnite crytals. Fracture: Subconchoidal to irregular; brittle; thin prisms are slightly flexible. Hardness: 2.0 Specific Gravity: 4.6 Luminescence: None Distinctive Features and Tests: Best field identification features are softness; flexibility of thin prisms; and long, bladed and acicular crystal habits. Visually similar to bismuthinite [bismuth trisulfide, Bi2S3], which has considerably more density. Dana Classification Number: 2.11.2.1 NAME Stibnite, pronounced STIBB-nite, derives from stibi, the Greek name for the mineral. Stibnite has formerly been referred to as “antimonite,” “antimonide,” “sulfur antimonide,” “sulfur antimony,” “antimony glance,” “gray antimony,” “gray antimony ore,” “kohl,” “stibi,” “stimmi,” “stibium,” “lupus metallorum,” “platyophthalmite,” and “speissglas.” In current European mineralogical literature, stibnite appears as stibnit, stibnita, and stibnine. Antimony, pronounced ANN-tih-mow-nee, stems from the Latin word for the metal, antimonium, which means “opposed to solitude,” in allusion to its rare occurrence as a native element.