Ecology and Biological Control of Verticillium Dahliae by L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uitnodiging Van De Werkgroep Wandelnetwerk Midden-Drenthe: Online Bijeenkomst 11 November 19.30 Uur Over De Werving Van Lokale Vrijwilligers
UITNODIGING VAN DE WERKGROEP WANDELNETWERK MIDDEN-DRENTHE: ONLINE BIJEENKOMST 11 NOVEMBER 19.30 UUR OVER DE WERVING VAN LOKALE VRIJWILLIGERS Doel van deze bijeenkomst is de contactpersonen uit de dorpen voor het wandelen te informeren over het werven van lokale vrijwilligers voor het controleren van het nieuwe wandelnetwerk. Het Recreatieschap Drenthe start met het werven van vrijwilligers voor het nieuw aanlegde wandelnetwerk in Midden-Drenthe (fase 1). Het is de bedoeling deze vrijwilligers zoveel mogelijk lokaal te werven, via de contactpersonen uit de dorpen of Verenigingen van Plaatselijk belang. Tijdens de bijeenkomst wordt de werkwijze om lokale vrijwilligers te werven toegelicht. Ook wordt er verteld wat Recreatieschap Drenthe verwacht van de vrijwilligers, welke werkzaamheden zij verrichten en hoe u vrijwilligers uit uw dorp of omgeving kunt aanmelden. Wanneer De bijeenkomst vindt plaats op woensdag 11 november vanaf 19.30 uur. Let op: het is een online bijeenkomst. De link naar de bijeenkomst wordt vooraf gestuurd aan de personen die zich hebben aangemeld. Voor wie - Contactpersonen voor het wandelen uit de dorpen en/of bestuursleden van Verenigingen voor Dorpsbelangen in het gebied waar het wandelnetwerk onlangs is aangelegd. - Denk aan de Broekstreek, Bruntinge, Drijber, Elp-Zuidveld, Eursinge (Westerbork), Holthe/Lieving, Nieuw Balinge, Smalhorst, Spier, Orvelte, Wijster, Witteveen, Zwiggelte en Westerbork. - Eén evt. twee personen per dorp kunnen zich aanmelden. Aanmelden U kunt zich aanmelden voor deze bijeenkomst door een bericht te sturen naar Ingeborg de Groot, onder vermelding van uw naam en het dorp dat u vertegenwoordigt. Het e-mailadres is: [email protected]. Voor vragen kunt u ook een bericht sturen of bellen op T. -

Orvelte-K4.Pdf
ROUTE K4 Knapzakroute Orvelte 17 km wandelen door onbekend Drenthe FOTO: SAKE ELZINGA Welkom in Orvelte gaat wandelen in en rond het monumen- U tendorp Orvelte. Slenter over de keien- straatjes van het dorp en maak in het informatiecentrum kennis met de geschiedenis van Orvelte. Bekijk de oude boerderijen met hun ouderwetse boerenerven en prachtige dorpsweitjes. Maar liefst zeventien Orvelter boerderijen staan op de monumentenlijst. De omgeving van Orvelte is minstens zo interessant, omdat daar net als in het dorp ook de moderne tijd grotendeels aan voor- bijgegaan lijkt. U merkt het op de Noordes van FOTO: SAKE ELZINGA SAKE FOTO: Orvelte met z’n kleine akkers en aan de rand van het dorp bij de groenlandjes die omzoomd zijn door houtwallen. Aan de overkant van het Oranjekanaal voelt u op het Orvelterzand en rond de Meeuwenplassen nog iets van de ongereptheid van het voormalige Ellertsveld. U komt op de plaats waar eens het dorp Orvelterveen gelegen moet hebben en u wandelt kilometers door een weinig bezocht deel van de Staatsbossen. START 2 KAART Start Tik op de nummers voor informatie Algemene informatie 4 Hoe werkt het? Uitleg over de route en praktische zaken 5 Routekaart + beschrijving 6 Orvelte, om te beginnen... 16 Het landschap van Orvelte 19 Informatie onderweg 24 De pdf geeft de beste gebruikservaring in Acrobat Reader of iBooks. START 3 KAART Algemene informatie Startpunt Café Warmolts, Schoolstraat 2, 9441 PE Orvelte. Auto (betaald) parkeren op één van de twee parkeerplaatsen aan de rand van het dorp. U kunt allen met pinpas betalen. Routebeschrijving Google naar de parkeerplaats. -

Groenbeleidsplan 2016-2025 Bijlagen Definitief Februari 2016
Groenbeleidsplan Uitwerkingen per kern Groenbeleidsplan 2016-2025 Bijlagen Definitief februari 2016 Groenbeleidsplan Uitwerkingen per kern 2016-2025 Inhoud Toelichting .................................................................................................................................. 3 Hoeveelheden ......................................................................................................................... 3 Kaarten toelichting ................................................................................................................. 4 Dorpen en landschap in Midden-Drenthe .............................................................................. 6 Beilen .......................................................................................................................................... 8 Westerbork ............................................................................................................................... 21 Smilde ....................................................................................................................................... 26 Bovensmilde ............................................................................................................................. 28 Hoogersmilde............................................................................................................................ 30 Hooghalen ................................................................................................................................. 31 Wijster -

Landschapsbeleidsplan 2012 Gemeente Midden-Drenthe
Landschapsbeleidsplan 2012 Gemeente Midden -Drenthe Landschapsbeleidsplan Midden-Drenthe September 2012, Gemeente Midden Drenthe VOORWOORD De gemeente Midden-Drenthe is een landelijke gemeente met veel buitengebied. De verschillende landschapstypen zijn een gevolg van de natuurlijke ontstaansgeschiedenis van het gebied en het gebruik door de mens. Door aandachtig naar het landschap te kijken kan men veel te weten komen over de geschiedenis van het landschap. En door de verschillen in het landschap zichtbaar te houden kan een grote tijdsdiepte in het landschap worden beleefd. De tijdsdiepte samen met de verschillende landschapstypen, die zorgen voor een afwisselend landschap. De hoofdrol is nog altijd weggelegd voor de landbouw. Natuurgebieden worden doorsneden door fiets- en wandelpaden en zijn daardoor goed ontsloten voor de inwoners, recreanten en toeristen in deze gemeente. De combinatie van dit alles maakt Midden-Drenthe niet alleen een aantrekkelijke gemeente om te recreëren, maar ook om te wonen. In 2000 is de eerste versie van dit landschapsbeleidsplan opgesteld, in de afgelopen tien jaar is er veel veranderd in de visie op landschap. Zo worden cultuurhistorie, archeologie en aardkundige waarden nu gezien als een belangrijk onderdeel van het landschap. Ook zijn thema’s als duurzaamheid en ecologisch beheer steeds meer aan de orde. Daarnaast is er meer onzekerheid over de realisatie en financiering van het landschap en natuur in verband met grootschalige bezuinigingen. In 2011 is besloten het plan te actualiseren. In dit plan worden een aantal belangrijke punten genoemd, die bij onderhoud en grotere projecten in het landschap meegenomen kunnen worden. De bedoeling is dat steeds wanneer er ruimte is binnen een project één of een aantal van deze punten uit te voeren en zo het landschap nog mooier te maken. -

En Evenementenverordening Gemeente Midden-Drenthe
Verordening vastgesteld: 30 maart 2000 Laatste wijziging: 20 december 2007 In werking getreden: 22 december 2007 Festiviteiten- en evenementenverordening gemeente Midden-Drenthe AFDELING 1 : BEGRIPSBEPALINGEN Artikel 1 In deze verordening wordt verstaan onder: a. inrichting : elke door de mens bedrijfsmatig of in een omvang alsof zij bedrijfsmatig was, ondernomen bedrijvigheid die binnen een zekere begrenzing pleegt te worden verricht; b. houder van een inrichting : degene die als eigenaar, bedrijfsleider, beheerder of anderszins een inrichting drijft; c. bevoegd gezag : de door burgemeester en wethouders dan wel door de burgemeester aangewezen ambtenaren van de gemeente Midden-Drenthe en de Regiopolitie voor het uitvoeren van controlewerkzaamheden inzake de naleving van de aan deze verordening verbonden voorschriften; d. collectieve festiviteit : festiviteit die niet specifiek aan één of een klein aantal inrichtingen is verbonden, zoals Koninginnedag, carnaval, kermis, etc.; e. incidentele festiviteit : festiviteit die verbonden is aan één of een klein aantal inrichtingen of wordt georganiseerd door één organiserende instantie, zoals de viering van een jubileum, etc.; f. evenement : elke voor publiek buiten de daartoe ingerichte inrichtingen toegankelijke festiviteit, grootschalige sportwedstrijd, auto- of motorcrosswedstrijd, optochten, georganiseerd vuurwerk en alle overige tot vermaak en recreatie bedoelde activiteiten met uitzondering van: - markten als bedoeld in de Gemeentewet, - kansspelen als bedoeld in de Wet op kansspelen en - betogingen, samenkomsten en vergaderingen als bedoeld in de Wet openbare manifestaties. Onder het begrip evenement wordt mede verstaan een herdenkingsplechtigheid; g. bebouwde kom : de bebouwde kom waarvoor de gemeenteraad de grenzen hebben vastgesteld krachtens artikel 20a van de Wegenverkeerswet 1994; h. deellocatie A : het centrum van Beilen en de dorpskern van Westerbork als aangegeven in de bij deze verordening behorende figuren 1 en 2; i. -

Presentatie Martin Van Dijken Landschapsarchitect
Brede Kijk op het Oude Diep 18 april 2019 presentatie Verlengde Middenraai Landschap van Velden en Lange Lijnen 1 Beilen Beil erstroom Makkum Ter Horst Holthe studiegebied Linthorst Homankanaal N381 Garminge Bruntinge Spier Wijster Witteveen Balinge Dwingelderveld Mantinge VAM-kanaal A28 Drijber 4.2c Middenraai Ruiner Aa invoegen luchtfoto met toponiemen ep e Di ud O Verlengde Middenraai Pesse Mekelermeer Nieuw Balinge Boswachterij Gees Stuifzand Tiendeveen Nieuweroord Hoogeveen Noordseschut A37 2 oriëntatie 3 Saalien 238.000 - 126.000 BC 4 Saalien vindplaatsen/restanten 238.000 - 126.000 BC • basisreliëf: Hondsrug, rug Zuidwolde en het keileemplateau ('Drents Plateau') • stenen en sedimenten van Scandinavische oorsprong in de ondergrond 5 Weichselien / Oude Steentijd (laat Pleistoceen) 150.000 - 10.000 BC 6 Weichselien / Oude Steentijd (laat Pleistoceen) Vindplaatsen/restanten 150.000 - 10.000 BC • beekdalen Oude Diep • droogdal bij Stuifzand • kampementen bij Boerveense Plassen, twee bij Hoogeveen (nu knooppunt Hooge- veen en de wijk Trasselt) • vuursteenvondsten bij Spier en Kraloo uit 12000 BC 7 Weichselien / Oude Steentijd (laat Pleistoceen) 150.000 - 10.000 BC 8 Weichselien / Oude Steentijd (laat Pleistoceen) 150.000 - 10.000 BC 9 Eind Weichselien / begin Holoceen / Mesolithicum 9600 - 6000 BC 10 Eind Weichselien / begin Holoceen / Mesolithicum Vindplaatsen/restanten 9600 - 6000 BC • Kano van Pesse, de oudste boot ter wereld (8000 BC) • haardkuilen op Fluitenberg en Noordseschut • het Mantingerbos op deze plek al 8000 jaar, restant van oorspronkelijke oerbos. • dekzandruggen en -vlakten verspreid door het gebied 11 9600 - 6000 BC 12 Neolithicum - Nieuwe Steentijd 4200 - 2000 BC 13 Neolithicum - Nieuwe Steentijd 4200 - Vindplaatsen/restanten 2000 BC • vondsten rond Pesse • strijdbijl in Pesserma • restveen bij Stuifzand, langs de Middenraai, Hullenzand, Mantingerzand, Lentsche Veen en andere veenrestanten. -

Veldnamen Broekstreek; Mantinge, Balinge, Garminge, Bruntinge Tot Wijster, Drijber
Veldnamen Broekstreek; Mantinge, Balinge, Garminge, Bruntinge tot Wijster, Drijber Verklaring naam Verklaring naam Veldnaam In omgeving van deel 1 deel 2 toelichting/bijzonderheden 1 Achterste Esch Mantinge achteraan / verderop es landbouwgrond gelegen 2 Achterste Knijpe (Kniepe) Mantinge achteraan / verderop smal afgeknepen heideveld van dorp uit gezien, op kaart 1899 gelegen aan de rand van ontginning. als heideveld. Nog niet ontgonnen Ook stuw om op hogere grond water vast te houden 3 Achterste veld Nieuw Balinge achteraan / verderop (voormalige)woestegrond, links van de Mantingerweg gelegen heide met zandverstuivingen 4 Balinger Esch Balinge bij Balinge es landbouwgrond 5 Balingerzand Mantinge dichter bij Balinge dan woestegrond, heide, vanuit Mantinge links van Mantinge jeneverbesstrueelen met Achterste Esch vennen en zandverstuivingen 6 Boekweitenplas (op Bruntinge boekweit ven/plas op het Scharreveld Scharreveld) 7 Bruntinger Binnenveld Bruntinge bij Bruntinge (voormalige)woestegrond, nu landbouw en grasland heide met zandverstuivingen 8 Bruntinger Bos Bruntinge bij Bruntinge bos deel van het beekdal vanaf Garminge 9 Bruntinger Esch Bruntinge bij Bruntinge es landbouwgrond 10 Bruntingerweiden Bruntinge bij Bruntinge weide gebied deel van het beekdal van het Oude Diep vanuit het Mantingerbos en -weiden 11 De Hof Balinge tuin en akkerbouw boerderij met (groente) hoek Heirweg/ Mepscheren tuinen; verwijst meestal naar een bisschoppelijk hof; Wierenga 12 De Hullen Drijber tussen Mantinge en nat gebied, broekbos, bovenloop vanaf Hullendijk -

Monumenten in Nederland. Drenthe
Monumenten in Nederland. Drenthe Ronald Stenvert, Sabine Broekhoven, Saskia van Ginkel-Meester, Chris Kolman en Redmer Alma bron Ronald Stenvert, Sabine Broekhoven, Saskia van Ginkel-Meester, Chris Kolman en Redmer Alma, Monumenten in Nederland. Drenthe. Rijksdienst voor de Monumentenzorg, Zeist / Waanders Uitgevers, Zwolle 2001 Zie voor verantwoording: http://www.dbnl.org/tekst/sten009monu07_01/colofon.php © 2010 dbnl / Ronald Stenvert, Sabine Broekhoven, Saskia van Ginkel-Meester, Chris Kolman en Redmer Alma i.s.m. schutblad voor Ronald Stenvert, Sabine Broekhoven, Saskia van Ginkel-Meester, Chris Kolman en Redmer Alma, Monumenten in Nederland. Drenthe 2 Rolde, Herv. kerk, interieur (1972) Ronald Stenvert, Sabine Broekhoven, Saskia van Ginkel-Meester, Chris Kolman en Redmer Alma, Monumenten in Nederland. Drenthe 4 Ansen, esdorp vanuit het zuidoosten Ronald Stenvert, Sabine Broekhoven, Saskia van Ginkel-Meester, Chris Kolman en Redmer Alma, Monumenten in Nederland. Drenthe 6 Echten, Huis te Echten, duifhuis (1982) Ronald Stenvert, Sabine Broekhoven, Saskia van Ginkel-Meester, Chris Kolman en Redmer Alma, Monumenten in Nederland. Drenthe 7 Voorwoord In de reeks Monumenten in Nederland, een gezamenlijk initiatief van de Rijksdienst voor de Monumentenzorg en Waanders Uitgevers, is dit het zevende, aan de provincie Drenthe gewijde deel. Het geeft een geïllustreerd overzicht van de monumenten in deze provincie en is in de eerste plaats bedoeld als beknopt naslagwerk voor een breed publiek. Als bron van informatie is het bruikbaar voor zowel de wetenschappelijk geïnteresseerde lezer als voor degene die vanuit een cultuurhistorische of toeristische belangstelling kort en bondig geïnformeerd wil worden. De reeks Monumenten in Nederland geeft een breed overzicht van de cultuurhistorisch meest waardevolle objecten en structuren. -

Nieuws Uit De Broekstreek
DE BROEKER NIEUWS UIT DE BROEKSTREEK www.broekstreek.nl Jaargang 34 nummer 3 Redactie: Marga Meppelink, Steendervalsweg 29, Mantinge, tel. 552456 Jorien Meppelink, Wijsterseweg 8b, Mantinge, tel. 552290 Jan van der Veen, Mantingerdijk 10, Mantinge, tel. 552565 Foto titelpagina: John Nycolaas Kopie voor de volgende Broeker kan worden ingeleverd UITERLIJK: 27 november 2013 Bij: Jorien Meppelink, Wijsterseweg 8b, Mantinge, tel. 552290 Bij voorkeur via e-mail: [email protected] Rooster voor het rondbrengen van de Broeker: (graag eventuele wijzigingen in de samenstelling van het bestuur doorgeven aan Marga Meppelink, dit in verband met het rondbrengen van de Broeker) Deze keer: de Soos Volgende keer: Kalverjasclub Westerbork: Gea Beuker Drukker: Koningin van Frankrijk [email protected] Agenda t'Broekhoes Woensdag 6 november: Soos 14.00 uur. Maandag 11 november: Vergadering Db. Plaatselijk Belang 20.00 uur. Dinsdag 12 november: Vergadering Agrarische Natuurver. Drenthe 20.00 uur. Woensdag 20 november: Toneelavond Soos 20.00 uur. Vrijdag 22 november: Buurt Academie Foto tentoonstelling 20.00 uur. Zaterdag 23 november: Foto tentoonstelling Zondag 24 november: Foto tentoonstelling Maandag 25 november: Buurt Academie 20.00 uur. Woensdag 27 november: Vrouwen van nu 19.45 uur. Oud papier wordt elke eerste zaterdag van de maand opgehaald, tenzij anders vermeld. De Praotiesmaoker Praotiesmaoker vindt het jammer dat er of de auto’s op de oprit de grond in steeds maor weer een onneudig gaon. De vraogsteller vindt het maor probleem maokt wordt. In de niks dat hekwerk in de berm van de Broekstreek weten wij niet beter of wij straot; dat de gemeente dit toelaot. spreken van het Mantingerzand. -

Landschapsadvies: Kaart
Gebiedsbestemmingen Essen en oude veldontginningen Essen Bovensmilde Oude veldontginningen (kampontginningen) Beekdalen Beekdalen I Beekdalen II Jonge veld- en veenontginningen Smilde Jonge veldontginningen Veenontginningen Bossen en velden Onder ‘Overige gebieden’ (functioneel) vallen de gebiedsbestemmingen P.M. ‘Bossen’ en ‘Velden’ (landschapseenheden). De bestemming en begrenzing Hooghalen moet nader worden bepaald op grond van de aanwijzing / verwerving van 1 natuurgebieden, huidige eigendom- en beheerssituatie, ontwikkelingsdoel. 2 4 Oranje 3 Hoogersmilde 3 5 2 Hijken 1 1 Elp 6 1 2 2 Klatering-Alting Zwiggelte 1 1 3 3 Brunsting 4 Beilen 3 2 Westerbork 1 1 1 Orvelte 1 2 Holthe-Lieving-Makkum 3 4 2 3 2 Terhorst 1 1 2 3 Garminge 6 2 3 1 4 Wijster Witteveen Spier Bruntinge 4 Balinge 5 7 Mantinge 2 1 1 Drijber 2 Nieuw Balinge Hooghalen Zwiggelte 1. De gronden noordelijk langs de Nijlanden zijn te 1. Dit stuk grond van de Eekhorsten behoort ook tot de beschouwen als kampontginningen. bovenloop van de Leek en is dus bij Beekdalen II 2. Hetzelfde geldt voor het Nijland zuidelijk, terwijl ondergebracht. het voor de Westkampen kan worden betwijfeld 2. Dit stuk grond noordelijk van het Oranjekanaal 3. De Holtes moet duidelijker begrensd worden naar behoort tot de Jonge veldontginningen en niet tot de Jonge veldontginningen. Beekdalen II. 3. De begrenzingen zijn hier wat aangepast, gekoppeld Hijken aan landschapselementen. 1. Een stukje bovenloop van de Vorrelveense Lake 4. Een groot stuk grond behoort tot de Jonge (noordelijk van N381) behoort tot Beekdalen II. veldontginningen. Holthe-Lieving-Makkum Spier 2. De Westerkamp bij Hijken is begrensd als es, 1. -
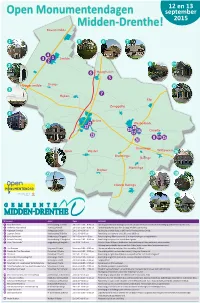
Open Monumentendagen Midden-Drenthe!
12 en 13 Open Monumentendagen september Midden-Drenthe! 2015 Bovensmilde 1 5 6 7 4 3 1 Smilde 2 2 8 t/m 6 Hooghalen 5 Hoogersmilde Oranje 15 3 7 Hijken Elp Zwiggelte 4 Beilen 21 Westerbork 21 22 19 18 17 16 Orvelte 23 8 t/m 15 20 Garminge 22 Spier Wijster Witteveen 16 Bruntinge Balinge Mantinge Drijber 23 17 18 Nieuw Balinge 19 20 Monument Adres Open Activiteit 1 Koepelkerk Smilde Veenhoopsweg 13 Smilde Zat en zon 11.00 – 16.00 uur Opening Open Monumentendagen om 11.00 uur door wethouder Gert Jan Bent. Bezichtiging ambachten restauratie kerk. 2 Graftombe Rebenscheidt Tramweg 14 Smilde Zat en zon 11.00 – 16.00 uur Toelichting graftombe door fam. de Jonge. Melden bij de woning. 3 Klipperaak Femmigje Aanlegsteiger Smilde Zat 11.00 – 16.00 uur Bezichtiging varend erfgoed uit 1907 van Sail Amsterdam naar Smilde. 4 Logement Smilde Veenhoopsweg 58 Smilde Zat 11.00 – 16.00 uur Rondleiding door bewoner van 11.00 uur tot 16.00 uur. 5 Kamp Westerbork Schattenberg 4 Zwiggelte Zat 12.00 uur en 14.00 uur Rondleiding langs Rijksmonumenten (o.a. waterzuivering en aardappelkelder). 6 Saksische boerderij Boermarkeweg 11 Hooghalen Zat en zon 11.00 – 16.00 uur Rondleiding over perceel en in pand door eigenaar. 7 Huize “Dennenrode” Zwiggelterweg 4 Hooghalen Zon 10.00 - 16.00 uur Kunst in Galerie Wildevuur. Beeldentuin: kinderworkshop schilderij maken met natuurvondsten. Verloting bronzen beeldje kunstenaar Piets Althuis (onder nieuwe leden Natuurmonumenten). 8 Huis Brandaan Brugstraat 2 Orvelte Zat en zon 11.00 – 17.00 uur Huis van vertelkunst en verhalen. -

Hedendaagse Veldnamen Broekstreek; Mantinge, Balinge, Garminge, Bruntinge Tot Wijster, Drijber, Nieuw Balinge En Meppen
Hedendaagse Veldnamen Broekstreek; Mantinge, Balinge, Garminge, Bruntinge tot Wijster, Drijber, Nieuw Balinge en Meppen Bronnen[ M.S = M. Schönfeld / J.W = J. Wieringa / A.H.B = A.H. Booij / T.S - Theo Spek / H.E - Hans Elerie [ DW = Drents woordenboek - http://woorden.huusvandetaol.nl/ - ED - Encyclopedie Drenthe Online / GTB - Geintegreerde Taalbank - http://gtb.inl.nl Verklaring naam Verklaring naam In Nummer Veldnaam omgeving deel 1 deel 2 toelichting/bijzonderheden van 1 Achterste Es Mantinge achteraan / verderop gelegen es landbouwgrond, kamppodzolgronden Achterste Kniepe Mantinge achteraan gelegen vanuit het dorp smal afgeknepen heideveld aan de rand van van dorp uit gezien, op kaart 1899 als 2 (Knijpe) gezien, ligt tussen de Steenkamp Es de es ontginning. (Ook stuw om op hogere heideveld. Nog niet ontgonnen. Landhek met en de Achterste Es grond water vast te houden?) naam Achterste Mantinge achteraan / verderop gelegen (smalle) weides in beekdalen vaak ook aan weerszijde van het Binnenveld. Stukken omheind (met houtwallen, elzen) Landhekken met naam Achterste veld Nieuw Balinge achteraan / verderop gelegen (voormalige) woestegrond, heide met links van de Mantingerweg 3 zandverstuivingen 4 Balinger Es Balinge bij Balinge es landbouwgrond Balingerzand Mantinge dichter bij Balinge dan Mantinge woestegrond, heide, jeneverbesstrueelen vanuit Mantinge links van Achterste Esch 5 met vennen en zandverstuivingen Boekweitenplas Bruntinge boekweit ven/plas op het Scharreveld 6 Bruntinger Bruntinge bij Bruntinge (voormalige)woestegrond, heide