ABSTRACT Title of Dissertation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dynamics of Woodpecker – Common Starling Interactions: a Comparison of Old World and New World Species and Populations
Ornis Hungarica 2016. 24(1): 1–41. DOI: 10.1515/orhu-2016-0001 Dynamics of Woodpecker – Common Starling interactions: a comparison of Old World and New World species and populations Jerome A. JACKSON1* & Bette J. S. JACKSON2 Received: May 03, 2016 – Accepted: June 10, 2016 Jerome A. Jackson & Bette J. S. Jackson 2016. Dynamics of Woodpecker – Common Starling interactions: a comparison of Old World and New World species and populations. – Ornis Hun- garica 24(1): 1–41. Abstract Woodpecker species whose cavities are most usurped by Common Starlings (Sturnus vulgaris) are widespread and generalists in their use of habitats. These include primarily woodpeckers that are similar in size to or slightly larger than the starling – such as the Great-spotted Woodpecker (Dendrocopos major) of Eurasia and the Northern Flicker (Colaptes auratus) and Red-bellied (Melanerpes carolinus) and Red-headed (M. erythrocephalus) Woodpeckers of North America. Usurpation occurs primarily in human-dominated urban, suburban and exurban habitats with pastures, sports fields and other open areas that serve as prime feeding habi tats for starlings. Starlings prefer high, more exposed cavities with a minimal entrance diameter relative to their body size. Usurpation success depends on timing – optimally just as a cavity is completed and before egg-lay- ing by the woodpeckers. Starlings likely reduce woodpecker populations in more open, human-dominated habi- tats. Woodpecker habitat losses and fragmentation are more serious problems that enhance habitat quality for star- lings and reduce habitat quality for most woodpeckers. The only woodpeckers that might become in danger of extinction as a primary result of starling cavity usurpation are likely island species with small populations. -

The Journal of Caribbean Ornithology
THE J OURNAL OF CARIBBEAN ORNITHOLOGY SOCIETY FOR THE C ONSERVATION AND S TUDY OF C ARIBBEAN B IRDS S OCIEDAD PARA LA C ONSERVACIÓN Y E STUDIO DE LAS A VES C ARIBEÑAS ASSOCIATION POUR LA C ONSERVATION ET L’ E TUDE DES O ISEAUX DE LA C ARAÏBE 2005 Vol. 18, No. 1 (ISSN 1527-7151) Formerly EL P ITIRRE CONTENTS RECUPERACIÓN DE A VES M IGRATORIAS N EÁRTICAS DEL O RDEN A NSERIFORMES EN C UBA . Pedro Blanco y Bárbara Sánchez ………………....................................................................................................................................................... 1 INVENTARIO DE LA A VIFAUNA DE T OPES DE C OLLANTES , S ANCTI S PÍRITUS , C UBA . Bárbara Sánchez ……..................... 7 NUEVO R EGISTRO Y C OMENTARIOS A DICIONALES S OBRE LA A VOCETA ( RECURVIROSTRA AMERICANA ) EN C UBA . Omar Labrada, Pedro Blanco, Elizabet S. Delgado, y Jarreton P. Rivero............................................................................... 13 AVES DE C AYO C ARENAS , C IÉNAGA DE B IRAMA , C UBA . Omar Labrada y Gabriel Cisneros ……………........................ 16 FORAGING B EHAVIOR OF T WO T YRANT F LYCATCHERS IN T RINIDAD : THE G REAT K ISKADEE ( PITANGUS SULPHURATUS ) AND T ROPICAL K INGBIRD ( TYRANNUS MELANCHOLICUS ). Nadira Mathura, Shawn O´Garro, Diane Thompson, Floyd E. Hayes, and Urmila S. Nandy........................................................................................................................................ 18 APPARENT N ESTING OF S OUTHERN L APWING ON A RUBA . Steven G. Mlodinow................................................................ -

Distribution, Ecology, and Life History of the Pearly-Eyed Thrasher (Margarops Fuscatus)
Adaptations of An Avian Supertramp: Distribution, Ecology, and Life History of the Pearly-Eyed Thrasher (Margarops fuscatus) Chapter 6: Survival and Dispersal The pearly-eyed thrasher has a wide geographical distribution, obtains regional and local abundance, and undergoes morphological plasticity on islands, especially at different elevations. It readily adapts to diverse habitats in noncompetitive situations. Its status as an avian supertramp becomes even more evident when one considers its proficiency in dispersing to and colonizing small, often sparsely The pearly-eye is a inhabited islands and disturbed habitats. long-lived species, Although rare in nature, an additional attribute of a supertramp would be a even for a tropical protracted lifetime once colonists become established. The pearly-eye possesses passerine. such an attribute. It is a long-lived species, even for a tropical passerine. This chapter treats adult thrasher survival, longevity, short- and long-range natal dispersal of the young, including the intrinsic and extrinsic characteristics of natal dispersers, and a comparison of the field techniques used in monitoring the spatiotemporal aspects of dispersal, e.g., observations, biotelemetry, and banding. Rounding out the chapter are some of the inherent and ecological factors influencing immature thrashers’ survival and dispersal, e.g., preferred habitat, diet, season, ectoparasites, and the effects of two major hurricanes, which resulted in food shortages following both disturbances. Annual Survival Rates (Rain-Forest Population) In the early 1990s, the tenet that tropical birds survive much longer than their north temperate counterparts, many of which are migratory, came into question (Karr et al. 1990). Whether or not the dogma can survive, however, awaits further empirical evidence from additional studies. -

Distribution, Probable Evolution, and Fossil Record of West Indian Woodpeckers (Family Picidae)
DISTRIBUTION, PROBABLE EVOLUTION, AND FOSSIL RECORD OF WEST INDIAN WOODPECKERS (FAMILY PICIDAE) ALEXANDER CRUZ Department of Biology University of Colorado Boulder, Colorado 80302 R ESUMEN : La familia Picidae (carpinteros) esta representada en la fauna de las Antillas por dote especies vivientes y dos especies fosiles. Las primeras estan comprendidas en dos generos endemicos y seis generos de distribution mas amplia. Las segundas constan de un genero conocido y otro especimen de afinidad desconocida. Los carpinteros estan mejor representados en Cuba, donde hay cinco especies residentes en comparacion con las Antillas Menores, a excepcion de la Guadalupe, donde no hay especies residences. Durante la epoca glacial del Pleistocene el nivel del agua era inferior al actual y muchas zonas fueron expuestas. Durante esta epoca y posiblemente antes (Plioceno), la mayoria de la avifauna de las Antillas se derivo de las regiones continentals cercanas. Los carpinteros de las Antillas probablemente se originaron en tres diferentes regiones: Norte America, Centro America, y Sur America. HE family Picidae, whose fossil history Cayman, Gran Bahama, Abaco, and T dates back to the Lower Pliocene of Watling’s Island), Centurus radiolatus North America (Brodkorb, 1970) is re- (Jamaica), Centurus striatus (Hispaniola), presented in the West Indian faunal region Melanerpes portoricensis (Puerto Rico and (Fig. 1) by twelve living species, eleven Vieques), Melanerpes herminiero (Guada- resident and one migratory. These com- loupe), Colaptes auratus (Cuba and Grand prise two endemic genera (Nesoctites and Cayman), Colaptes (Nesoceleus) fernandi- Xiphiodiopicus) and six genera of a greater nae (Cuba), Xiphiodiopicus percussus (Cuba distribution (Colaptes, Melanerpes, Centu- and the Isle of Pines), Dendrocopos villosus rus, Sphyrapicus, Dendrocopos, and Cam- (New Providence, Andros, Grand Bahama pephilus). -

Bird Watcher's General Store
BIRD OBSERVER Carolina Wren © Barry Van Dusen, 1989 VOL. 18 NO. 6 DECEMBER 1990 BIRD OBSERVER VOL. 18 NO. 6 DECEMBER 1990 Editorial and Production Staff Dorothy R. Arvidson Theodore H. Atkinson Chere Bemelmans Associate Editor Brian E. Cassie Janet L. Heywood William E. Davis, Jr. Advisory Board Glenn d'Entremont Kathleen S. Anderson Herman H. D'Entremont James Baird H. Christian Floyd Alden G. Clayton Richard A. Forster Thomas W. French George W. Gove John C. Kricher Harriet E. Hoffman Ian C. T. Nisbet David E. Lange Bruce A. Sortie Simon A. Perkins Richeird K. Walton Wayne R. Petersen Martha Steele Corporate Officers Robert H. Stymeist William E. Davis, Jr., President Claudia Taylor Lee E. Taylor, Treasurer Lee E. Taylor H. Christian Floyd, Clerk Martha W. Vaughan BIRD OBSERVER{USPS 369-850) is published bimonthly, COPYRIGHT © 1990 by Bird Observer of Eastern Massachusetts, Inc., 462 Trapelo Road, Belmont, MA 02178, a nonprofit, tax-exempt corporation under section 501 (c)(3) of the Internal Revenue Code. Gifts to Bird Observer will be greatly appreciated and are tax deductible. POSTMASTER: Send address changes to BIRD OBSERVER, 462 Trapelo Road, Belmont, MA 02178. SUBSCRIPTIONS: $16 for 6 issues per calendar year, $30 for two years in the U. S. Add $2.50 per year for Canada and foreign. Single copies $3.00. An Index to Volumes 1-11 is $3. Back issues: inquire as to price and availabilify. CHANGES OF ADDRESS and subscription inquiries should be sent to Bird Observer Subscriptions, P. O. Box 236, Arlington, MA 02174. ADVERTISING: full page, $80; half page, $40; quarter page, $25. -

Boa, Epicrates Subflavus
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 2010 MOVEMENTS, ACTIVITY RANGE, HABITAT USE, AND CONSERVATION OF THE JAMAICAN (YELLOW) BOA, EPICRATES SUBFLAVUS Erika Elise Miersma The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Miersma, Erika Elise, "MOVEMENTS, ACTIVITY RANGE, HABITAT USE, AND CONSERVATION OF THE JAMAICAN (YELLOW) BOA, EPICRATES SUBFLAVUS" (2010). Graduate Student Theses, Dissertations, & Professional Papers. 684. https://scholarworks.umt.edu/etd/684 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. MOVEMENTS, ACTIVITY RANGE, HABITAT USE, AND CONSERVATION OF THE JAMAICAN (YELLOW) BOA, EPICRATES SUBFLAVUS by Erika Elise Miersma Bachelor of Science, Calvin College, Grand Rapids, MI, 2006 Thesis presented in partial fulfillment of the requirements for the degree of Master of Science in Resource Conservation, International Conservation and Development The University of Montana Missoula, MT Summer 2010 Miersma, Erika, M.S., Summer 2010 Resource Conservation Movements, Activity Ranges, Habitat Use, and Conservation of the Jamaican (Yellow) Boa, Epicrates subflavus Chair: Dr. Christopher Servheen The endemic Jamaican boa, Epicrates subflavus, was once common throughout Jamaica. This vulnerable species is now fragmented into small populations throughout the island due to habitat loss, introduced species, human persecution and poaching. -

Greater Antilles
Greater Antilles Jamaica, Cuba, Dominican Republic, and Puerto Rico (and Cayman Islands) Todies and Tyrants A Greentours Tour Report th th 27 November to 18 December 2014 Led by Paul Cardy Trip report written by Paul Cardy Introduction This ambitious tour of all the main Greater Antillean islands gives the chance to see a wealth of single island and regional endemic birds, butterflies and reptiles. Some 110 endemic birds were recorded, including all five of the world’s todys, endemic to the region. Our trip took us through five remarkably contrasting countries and cultures. Beautiful scenery, from the misty Blue Mountains of Jamaica, to the swamps of Cuba’s Zapata peninsula, Dominican Republic’s forested mountains, and the Guanica Dry Forest in Puerto Rico characterised the journey. A remarkably varied tour, illustrated by the Cuban example of watching Blue-headed Quail-Doves on forest trails in Zapata, and also experiencing the vibrancy of fascinating Old Havana. A feature was the incredible views we had of many rare endemic birds, such as Chestnut-bellied Cuckoo and both endemic parrots on Jamaica; Bee Hummingbird, Fernadina’s Flicker, and Zapata Wren on Cuba; Hispaniolan Woodpecker and Black-crowned Palm-Tanager in Dominican Republic; and Elfin Woods Warbler on Puerto Rico. There were some very special butterflies too such as Grand Cayman Swallowtail, Jamaican Monarch, two species of Anetia, Haitian Snout, Haitian Admiral, Cuban Emperor, Dusky Emperor, Cuban Lucinia, Cuban Dagger Tail, seven species of Calisto, and Haitian Pygmy Skipper. One area in Hispaniola, discovered on the previous visit, proved especially good for butterflies. -

Breeds on Islands and Along Coasts of the Chukchi and Bering
FAMILY PTEROCLIDIDAE 217 Notes.--Also known as Common Puffin and, in Old World literature, as the Puffin. Fra- tercula arctica and F. corniculata constitutea superspecies(Mayr and Short 1970). Fratercula corniculata (Naumann). Horned Puffin. Mormon corniculata Naumann, 1821, Isis von Oken, col. 782. (Kamchatka.) Habitat.--Mostly pelagic;nests on rocky islandsin cliff crevicesand amongboulders, rarely in groundburrows. Distribution.--Breedson islandsand alongcoasts of the Chukchiand Bering seasfrom the DiomedeIslands and Cape Lisburnesouth to the AleutianIslands, and alongthe Pacific coast of western North America from the Alaska Peninsula and south-coastal Alaska south to British Columbia (QueenCharlotte Islands, and probablyelsewhere along the coast);and in Asia from northeasternSiberia (Kolyuchin Bay) southto the CommanderIslands, Kam- chatka,Sakhalin, and the northernKuril Islands.Nonbreeding birds occurin late springand summer south along the Pacific coast of North America to southernCalifornia, and north in Siberia to Wrangel and Herald islands. Winters from the Bering Sea and Aleutians south, at least casually,to the northwestern Hawaiian Islands (from Kure east to Laysan), and off North America (rarely) to southern California;and in Asia from northeasternSiberia southto Japan. Accidentalin Mackenzie (Basil Bay); a sight report for Baja California. Notes.--See comments under F. arctica. Fratercula cirrhata (Pallas). Tufted Puffin. Alca cirrhata Pallas, 1769, Spic. Zool. 1(5): 7, pl. i; pl. v, figs. 1-3. (in Mari inter Kamtschatcamet -
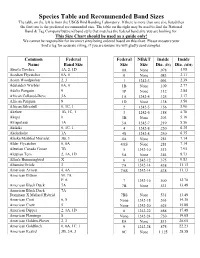
Species Table and Recommended Band Sizes the Table on the Left Is from the USGS Bird Banding Laboratory
Species Table and Recommended Band Sizes The table on the left is from the USGS Bird Banding Laboratory. If there is more than one size listed then the first one is the preferred recommended size. The table on the right may be used to find the National Band & Tag Company butt-end band style that matches the federal band size you are looking for. This Size Chart should be used as a guide only! We cannot be responsible for incorrect sizes being ordered based on this chart. Please measure your bird’s leg for accurate sizing, if you are unsure we will gladly send samples. Common Federal Federal NB&T Inside Inside Name Band Size Size Size Dia. (IN) Dia. (MM) Abert's Towhee 1A, 2, 1D 0A None .078 1.98 Acadian Flycatcher 0A, 0 0 None .083 2.11 Acorn Woodpecker 2, 3 1 1242-3 .094 2.39 Adelaide's Warbler 0A, 0 1B None .109 2.77 Adelie Penguin 9 1P None .112 2.84 African Collared-Dove 3A 1A 1242-4 .125 3.17 African Penguin 9 1D None .138 3.50 African Silverbill 0, 1C, 1 2 1242-5 .156 3.96 Akekee 1B, 1C, 1 3 1242-6 .188 4.78 Akepa 0 3B None .203 5.16 Akiapolaau 1A 3A 1242-7 .219 5.56 Akikiki 0, 1C, 1 4 1242-8 .250 6.35 Akohekohe 1A 4S 1242-8 .250 6.35 Alaska Marbled Murrelet 3B, 3 4A None .281 7.14 Alder Flycatcher 0, 0A 4AS None .281 7.14 Aleutian Canada Goose 7B 5 1242-10 .313 7.95 Aleutian Tern 2, 1A, 1D 5A None .344 8.73 Allen's Hummingbird X 6 1242-12 .375 9.53 Altamira Oriole 3 7A 1242-14 .438 11.13 American Avocet 4, 4A 7AS 1242-14 .438 11.13 American Bittern M: 7A F: 6 7 1242-16 .500 12.70 American Black Duck 7A 7B None .531 13.49 American -

Species Booklet
About NEPA The National Environment and Planning Agency (NEPA) is the lead government agency with the mandate for environmental protection, natural resource management, land use and spatial planning in Jamaica. NEPA, through the Town and Country Planning Authority and the Natural Resource Conservation Authority, operates under a number of statutes which include: The Town and Country Planning Act The Land Development and Utilization Act The Beach Control Act The Watershed Protection Act The Wild Life Protection Act The Natural Resources Conservation Authority Act. Vision NEPA’s vision is “for a Jamaica where natural resources are used in a sustainable way and that there is a broad understanding of environment, planning and development issues, with extensive participation amongst citizens and a high level of compliance with relevant legislation.” Mission “To promote Sustainable Development by ensuring the protection of the envi- ronment and orderly development in Jamaica through highly motivated staff performing at the highest standard.” A view of the Blue Mountains The agency executes its mandate through the development of environmental and planning policies; monitoring the natural resource assets and the state of Jamaica’s environment; enforcement of environmental and planning legislation; processing of applications for environmental permits and licences; preparing Town and Parish Development Plans and Parish Development Orders; providing environmental and land use database systems; advising on land use planning and development; public -

Bioone COMPLETE
BioOne COMPLETE Introduction to the Skeleton of Hummingbirds (Aves: Apodiformes, Trochilidae) in Functional and Phylogenetic Contexts Author: Zusi, Richard L., Division of Birds, National Museum of Natural History, P.O. Box 37012, Smithsonian Institution, Washington, D.C. 20013, USA Source: Ornithological Monographs No. 77 Published By: American Ornithological Society URL: https://doi.org/10.1525/om.2013.77.L1 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne's Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/ebooks on 1/14/2019 Terms of Use: https://bioone.org/terms-of-use Access provided by University of New Mexico Ornithological Monographs Volume (2013), No. 77, 1-94 © The American Ornithologists' Union, 2013. Printed in USA. INTRODUCTION TO THE SKELETON OF HUMMINGBIRDS (AVES: APODIFORMES, TROCHILIDAE) IN FUNCTIONAL AND PHYLOGENETIC CONTEXTS R ic h a r d L. Z u s i1 Division of Birds, National Museum of Natural History, P.O. -

A New Methodology to Use Color Spectral Data for Taxonomic, Phylogenetic, and Biogeographic Studies
A new methodology to use color spectral data for taxonomic, phylogenetic, and biogeographic studies. An example with three genera of lowland hummingbirds: Topaza, Anthracothorax, and Eulampis. Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Angela Schmitz Ornés aus München Bonn 2004 ii Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn 1. Referent: Prof. Dr. K.-L.Schuchmann 2. Referent: Prof. Dr. W. Böhme Tag der Promotion: Diese Dissertation ist auf dem Hochschulschriftenserver der ULB Bonn http://hss.ulb.uni- bonn.de/diss_online elektronisch publiziert iii Dedico este trabajo a mi hija ANDREA CAROLINA por ser mi motor de vida y fuente de inspiración ... iv Contents • Introduction …………………………………………………………………………….1 o Phylogenetic, systematic, and taxonomic bird studies …………………………3 o Hummingbirds: phylogeny and origin ….……………………………………..5 o Characteristics and distribution of the study taxa …...………….……………...7 o Research goals and structure of the study ……………………………………...9 • General methodology …………………………………………………………………13 o Specimens included in the study ……………………………………………...13 o Mappings ……………………………………………………………………...16 o Morphometric data ……………………………………………………………16 o Color measurements (spectral data) …………………………………………..16 o Data analyses …………………………………………………………………19 o Definition of preliminary pools for analysis ………………………………….20 • Methodology