A Microbiome-Driven Approach to Combating Depression During the COVID-19 Pandemic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spotlight and Hot Topic Sessions Poster Sessions Continuing
Sessions and Events Day Thursday, January 21 (Sessions 1001 - 1025, 1467) Friday, January 22 (Sessions 1026 - 1049) Monday, January 25 (Sessions 1050 - 1061, 1063 - 1141) Wednesday, January 27 (Sessions 1062, 1171, 1255 - 1339) Tuesday, January 26 (Sessions 1142 - 1170, 1172 - 1254) Thursday, January 28 (Sessions 1340 - 1419) Friday, January 29 (Sessions 1420 - 1466) Spotlight and Hot Topic Sessions More than 50 sessions and workshops will focus on the spotlight theme for the 2019 Annual Meeting: Transportation for a Smart, Sustainable, and Equitable Future . In addition, more than 170 sessions and workshops will look at one or more of the following hot topics identified by the TRB Executive Committee: Transformational Technologies: New technologies that have the potential to transform transportation as we know it. Resilience and Sustainability: How transportation agencies operate and manage systems that are economically stable, equitable to all users, and operated safely and securely during daily and disruptive events. Transportation and Public Health: Effects that transportation can have on public health by reducing transportation related casualties, providing easy access to healthcare services, mitigating environmental impacts, and reducing the transmission of communicable diseases. To find sessions on these topics, look for the Spotlight icon and the Hot Topic icon i n the “Sessions, Events, and Meetings” section beginning on page 37. Poster Sessions Convention Center, Lower Level, Hall A (new location this year) Poster Sessions provide an opportunity to interact with authors in a more personal setting than the conventional lecture. The papers presented in these sessions meet the same review criteria as lectern session presentations. For a complete list of poster sessions, see the “Sessions, Events, and Meetings” section, beginning on page 37. -

Bibliography
List Required by the Trafficking Victims Protection Reauthorization Act of 2005 Updated December 15, 2010 BIBLIOGRAPHY AFGHANISTAN - BRICKS 1. Altai Consulting Group, and ILO-IPEC. A Rapid Assessment on Child Labour in Kabul. Kabul, January 2008. 2. ILO. Combating Child Labour in Asia and the Pacific: Progress and Challenges. Geneva, 2005. 3. Save the Children Sweden-Norway. "Nangarhar, Sorkrhod: Child Labor Survey Report in Brick Making." Kabul, March 2008. 4. U.S. Department of State. Country Reports on Human Rights Practices - 2007. Washington, DC, March 11, 2008; available from http://www.state.gov/g/drl/rls/hrrpt/2007/100611.htm. 5. U.S. Department of State. Trafficking in Persons Report - 2006. Washington, DC, June 2006; available from http://www.state.gov/g/tip/rls/tiprpt/2006/. 6. U.S. Embassy- Kabul. reporting. December 1, 2007. 7. United Nations Foundation. U.N. Documents Child Labor Among Afghans, 2001. AFGHANISTAN - CARPETS 1. Afghanistan Independent Human Rights Commission. An Overview on Situation of Child Labour in Afghanistan Research Report. Kabul, 2006; available from http://www.aihrc.org.af/rep_child_labour_2006.pdf. 2. Altai Consulting Group and ILO-IPEC. A Rapid Assessment on Child Labour in Kabul. Kabul, January 2008. 3. Amnesty International. Afghanistan- Out of Sight, Out of Mind: The Fate of the Afghan Returnees. June 22, 2003; available from http://www.amnesty.org/en/library/info/ASA11/014/2003. 4. Chrobok, Vera. Demobilizing and Reintegrating Afghanistan’s Young Soldiers.Bonn International Center for Conversion, Bonn, 2005; available from http://www.isn.ethz.ch/isn/Digital- Library/Publications/Detail/?ots591=cab359a3-9328-19cc-a1d2- 8023e646b22c&lng=en&id=14372. -

Robson, Barbara TITLE Pashto Reader. INSTITUTION Center for Applied Linguistics, Washington, D.C
DOCUMENT RESUME ED 353 815 FL 020 896 AUTHOR Tegey, Habibullah; Robson, Barbara TITLE Pashto Reader. INSTITUTION Center for Applied Linguistics, Washington, D.C. SPONS AGENCY Office of International Education (ED), Washington, DC. PUB DATE 92 CONTRACT P017A10030 NOTE 226p.; For related documents, see FL 020 894-895. PUB TYPE Guides Classroom Use Instructional Materials (For Learner) (051) EDRS PRICE MF01/PC10 Plus Postage. DESCRIPTORS Advertising; Grammar; Instructional Materials; *Language Variation; Letters (Correspondence); News Media; *Pashto; Poetry; *Reading Materials; Uncommonly Taught Languages; Vocabulary; *Written Language IDENTIFIERS *Authentic Materials ABSTRACT This reader is the basic text for a set of instructional materials in Pashto. It consists of 45 authentic passages in Pashto script, each accompanied by background information, a vocabulary list, hints for scanning, comprehension exercises, and notes for detailed rereading. An introductory section offers study suggestions for the student. The passages are presented in 7 groups: essays; articles; stories; poetry; public writing (signs and advertising); letters and memoranda; and fractured Pashto. Each group is accompanied by an introduction and answers to comprehension questions. Additional jokes and anecdotes are included throughout the materials. (MSE) *********************************************************************** * Reproductions supplied by EDRS are the best that can be made * * from the original document. * *********************************************************************** -

Graduation Program Have Completed an Enriched Program of Study Through the Calhoun Honors College
CANDIDATES FOR THE DOCTOR’S DEGREE Jason W Osborne, Dean, Graduate School COLLEGE OF AGRICULTURE, FORESTRY COLLEGE OF BEHAVIORAL, SOCIAL AND AND LIFE SCIENCES HEALTH SCIENCES DOCTOR OF PHILOSOPHY DOCTOR OF PHILOSOPHY Entomology Human Factors Psychology Sofia Isabel Munoz ........................................Quito, Ecuador Drea Kevin Fekety ........................................Clearwater, FL Drew Morgan Link ............................................Bostic, NC Food Technology Drew Michael Morris ....................................Aberdeen, SD Mengmeng Zhao ..........................................Tianjin, China Industrial/Organizational Psychology Forest Resources Pamela Rose Farago ....................................... Wharton, NJ Jamie Marie Fynes ......................................McDonald, OH Gavin Douglas Blosser ........................................ Dillwyn, VA William S Kramer .................................Boynton Beach, FL Plant and Environmental Sciences International Family and Community Studies Nathan W Redding .....................................Greenville, SC Melody Jo Harper ........................................Lynchburg, VA Dhanuska Bandara Wijesinghe ...............Kandy, Sri Lanka Phillip Bryson Williams .....................................Neeses, SC Parks, Recreation and Tourism Management Wildlife and Fisheries Biology Zeynep A Gedikoglu ......................................Clemson, SC Ciera Marie Baird .............................................Pittston, PA Geoffrey Koome Riungu.................................Meru, -

(1.1.1) Curricula Developed /Adopted Have Relevance to the Local
Department of Management Studies (1.1.1) Curricula developed /adopted have relevance to the local/ national / regional/global developmental needs with learning objectives including program outcomes, program specific outcomes and course outcomes of all the programs offered by the University Organizations have to learn to survive, maintain a competitive edge and grow in fast-changing operating environments where individuals’ knowledge and its exploitation, coupled with the power of contemporary technologies, are critical to success. In developing effective solutions to real world problems, management practitioners need to adopt a holistic balance and fusion between the people-centred and techno-centric approaches to the subject. To respond to this reality of contemporary business, the management schools need to prepare students for the critical role they have to play in a highly dynamic business. It is in this context that the Department of Management Studies offers two years Masters in Business Administration (MBA), MBA (Financial Management), Masters in Tourism & Travel Management (MTTM) and five-year integrated MBA programme (IMBA). The programmes develop students’ managerial capabilities and competencies in their respective fields. The student learn through a multi-disciplinary approach to appreciate the scope, range and depth of management process, models, tools, techniques and their impact on organizational performance. Objectives of the Programmes The Programmes aim to: Provide intensive theoretical and practical knowledge of management. Provide an integrated perspective of management functioning along with a fair amount of exposure to real life cases/technical know-how. Enable students to become reflective practitioners in the area of management. Develop students’ operational and strategic capabilities and competencies in all functional areas of management and Develop research and critical thinking skills. -

Identity and Difference in a Muslim Community in Central Gujarat, India Following the 2002 Communal Violence
Identity and difference in a Muslim community in central Gujarat, India following the 2002 communal violence Carolyn M. Heitmeyer London School of Economics and Political Science PhD 1 UMI Number: U615304 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U615304 Published by ProQuest LLC 2014. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 F Declaration I certify that the thesis I have presented for examination for the MPhil/PhD degree of the London School of Economics and Political Science is solely my own work other than where I have clearly indicated that it is the work of others (in which case the extent of any work carried out jointly by me and any other person is clearly identified in it). The copyright of this thesis rests with the author. Quotation from it is permitted, provided that full acknowledgement is made. This thesis may not be reproduced without the prior written consent of the author. I warrant that this authorization does not, to the best of my belief, infringe the rights of any third party. -

People of Ghazni
Program for Culture & Conflict Studies www.nps.edu/programs/ccs Province: Kapisa Governor: Khoja Ghulam Ghous Abobakar Provincial Police Chief: Muhammad Ewaz Population Estimate: 364,900 Urban: 1,900 Rural: 363,0001 Area in Square Kilometers: 1,842 Capital: Mahmud-e-Eraqi Names of Districts: Hesa Dovon Kohistan, Hesa Aval Kohistan, Mahmud-e-Eraqi, Koh Band, Nijrab, Ala Sai, and Tag Ab Composition of Ethnic Groups: Religious Groups: Tribal Groups: Pashai, Population: Tajik, Pashtun, Majority Sunni Safi, Ghilzai, Kata & Nuristani Total # Mosques: Ashtu Nuristani 2,0292 Occupation of Population Major: Agriculture, labor, animals Minor: trade Crops/Farming/Livestock: Wheat, corn, fruit Cows, sheep, goats Literacy Rate Total: 32%3 Number of Educational Institutions: 994 Colleges/Universities: 1 Number of Security January: 2 March: 2 May: 1 Incidents, Jan-Jun 2007: 9 February: 1 April: 2 June: 1 Poppy (Opium) Cultivation: 2006: 282 ha 2007: 835 ha Agencies Active in Province: ACTION, ICRC, MSF, SCA, WFP, IAM, DACCAR Provincial Aid Projects:5 Total PRT Projects: 71 Other Aid Projects: 1100 Total Projects: 1171 Planned Cost: $8,332,821.48 Planned Cost: $8,739,979.64 Planned Cost: $17,072,801.12 Total Spent: $2,608,068.48 Total Spent: $5,475,974.00 Total Spent: $8,084,042.48 Transportation: Primary Roads: Tagab-Kabul Highway Health Facilities: Hospitals: 2 Clinics, etc.: 356 7 Primary Sources of Drinking Wells, rivers, karezes 22% Water/Availability of Potable Water: Rivers: Daria Panshjir runs along the Southwestern border of the province Significant Topographic The Northeastern districts contain the foothills of the Hindu Kush Features and some large forested areas while the Southwest is rockier and flatter towards the Daria Panshjir 1 Afghan Information Management Services, 2003-2004 Population Statistics, available from http://www.aims.org.af/ (accessed September 17, 2007). -
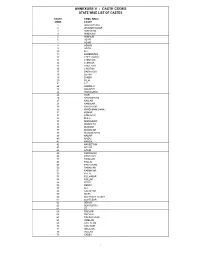
Annexure V - Caste Codes State Wise List of Castes
ANNEXURE V - CASTE CODES STATE WISE LIST OF CASTES STATE TAMIL NADU CODE CASTE 1 ADDI DIRVISA 2 AKAMOW DOOR 3 AMBACAM 4 AMBALAM 5 AMBALM 6 ASARI 7 ASARI 8 ASOOY 9 ASRAI 10 B.C. 11 BARBER/NAI 12 CHEETAMDR 13 CHELTIAN 14 CHETIAR 15 CHETTIAR 16 CRISTAN 17 DADA ACHI 18 DEYAR 19 DHOBY 20 DILAI 21 F.C. 22 GOMOLU 23 GOUNDEL 24 HARIAGENS 25 IYAR 26 KADAMBRAM 27 KALLAR 28 KAMALAR 29 KANDYADR 30 KIRISHMAM VAHAJ 31 KONAR 32 KONAVAR 33 M.B.C. 34 MANIGAICR 35 MOOPPAR 36 MUDDIM 37 MUNALIAR 38 MUSLIM/SAYD 39 NADAR 40 NAIDU 41 NANDA 42 NAVEETHM 43 NAYAR 44 OTHEI 45 PADAIACHI 46 PADAYCHI 47 PAINGAM 48 PALLAI 49 PANTARAM 50 PARAIYAR 51 PARMYIAR 52 PILLAI 53 PILLAIMOR 54 POLLAR 55 PR/SC 56 REDDY 57 S.C. 58 SACHIYAR 59 SC/PL 60 SCHEDULE CASTE 61 SCHTLEAR 62 SERVA 63 SOWRSTRA 64 ST 65 THEVAR 66 THEVAR 67 TSHIMA MIAR 68 UMBLAR 69 VALLALAM 70 VAN NAIR 71 VELALAR 72 VELLAR 73 YADEV 1 STATE WISE LIST OF CASTES STATE MADHYA PRADESH CODE CASTE 1 ADIWARI 2 AHIR 3 ANJARI 4 BABA 5 BADAI (KHATI, CARPENTER) 6 BAMAM 7 BANGALI 8 BANIA 9 BANJARA 10 BANJI 11 BASADE 12 BASOD 13 BHAINA 14 BHARUD 15 BHIL 16 BHUNJWA 17 BRAHMIN 18 CHAMAN 19 CHAWHAN 20 CHIPA 21 DARJI (TAILOR) 22 DHANVAR 23 DHIMER 24 DHOBI 25 DHOBI (WASHERMAN) 26 GADA 27 GADARIA 28 GAHATRA 29 GARA 30 GOAD 31 GUJAR 32 GUPTA 33 GUVATI 34 HARJAN 35 JAIN 36 JAISWAL 37 JASODI 38 JHHIMMER 39 JULAHA 40 KACHHI 41 KAHAR 42 KAHI 43 KALAR 44 KALI 45 KALRA 46 KANOJIA 47 KATNATAM 48 KEWAMKAT 49 KEWET 50 KOL 51 KSHTRIYA 52 KUMBHI 53 KUMHAR (POTTER) 54 KUMRAWAT 55 KUNVAL 56 KURMA 57 KURMI 58 KUSHWAHA 59 LODHI 60 LULAR 61 MAJHE -

Key Words- Caste, Occupation, Jajman, Patrons, Barter, Jati, Varna
INDIAN ANTHROPOLOGY Indian village as a unity and extension, village and caste Dr. Suninder Kaur HIG 914, Phase 2,Mohali, Chandigarh-160055 Introduction The Jajmani System The Varna Model Historical Background The Jati System Jati and their Relationship to Land The Untouchables The Dalits Birth based classification: India vis-à-vis other Nations Jati vis-a-vis Varna Egalitarian Societies Social Stratification in other parts of the world The Hindu Jajmani System Origin The Jajman-Kamin Relation Dynamics of rural society and the concept of social change Sanskritization Indian village as a unity and extension The Great Tradition and Little Tradition Universalisation and Parochialisation Durmont’s concept of Homo Hierarchicus Equality Social hierarchy subsequent to 1947 Independence Social Mobilization and Social Change Upward Mobility Caste system in contemporary society Social Stratification Practical Functionality of Caste System at Village level Indebtedness and Bonded labour Inter-Caste Relations Jajmani system in Contemporary Era Green revolution White Revolution Monetisation Bhakti movement Security of Livelihood and Employment The Declining Caste system Conclusions References Key Words Caste, Occupation, Jajman, Patron, Barter, Jati, Varna, Artisans, Kamin, Untouchable, Harijan, Client, Mobility, Hierarchy, Oppressed, Discrimination, Service, Endogamy, Exogamy, Dalit, kinship, polyandry, polygamy Introduction In India, for centuries, caste system and its sub division has been a very prominent segmentation of the society. The division of people into various varnas, jatis etc was basically on the basis of occupation. The work force was bifurcated into various castes as per their activity and skills. The skill and expertise was passed on from generations to generations whereby the profession ran in the family hierarchy and became the family occupation. -
![Lkseokj] Iqjojh 17] 2014@Ek?K 28] 1935 No](https://docslib.b-cdn.net/cover/5151/lkseokj-iqjojh-17-2014-ek-k-28-1935-no-3325151.webp)
Lkseokj] Iqjojh 17] 2014@Ek?K 28] 1935 No
jftLVªh laö Mhö ,yö&33004@99 REGD. NO. D. L. -33004/99 vlk/kj.k EXTRAORDINARY Hkkx I—[k.M 1 PART I—Section 1 izkf/dkj ls izdkf'kr PUBLISHED BY AUTHORITY la. 41] ubZ fnYyh] lkseokj] iQjojh 17] 2014@ek?k 28] 1935 No. 41] NEW DELHI, MONDAY, FEBRUARY 17, 2014/MAGHA 28, 1935 सामाDजक या य और अिधका@रता मंऽालय (सामाDजक याय और अिधका@रता Bवभाग) संकप ubZ fnYyh] 17 iQjojh] 2014 फाफाफा.फा ...संसंसंसं....120151201512015////05050505////201120112011----बीबीबीबी....सीसीसी ...II ...Ñ.ÑÑÑ जबAक मंडल आयोग क3 @रपोट मQ दोनU सूिचयU मQ शािमल जाितयU और समुदायU को समाAहत करके अ य Bपछड़े वग क3 सामा य के ि/य सूची और 26 रा यU और संघ रा य >ेऽU से संबंिधत अ य Bपछड़े वग क3 सामाय के ि/ य सूची को अनुबंधअनुबंध----11 मQ यथा िनAदं ट कया ण मंऽालय के संक पU के तहत अिधसूिचत Aकया गया था; और जबAक, रां श/ य Bपछड़ा वग आयोग (इसके बाद एनसीबी सी के प मQ संदिभत ) का गठन रांश/य Bपछड़ा वग आयोग अिधिनयम, 1993 (1993 का 27) क3 धारा 3 के अंतगत Aकया गया था और इसे नाग@र कU के Aकसी वग को सूिचयU मQ Bपछड़ा वग के प मQ शािमल करने संबंधी अनुरोध क3 जांच करने और ऐसी सूची मQ Aकसी Bपछड़े वग के अित समावेशन एवं अ प समावेशन क3 िशकायतU को सुनने तथा के ि/ य सरकार को सलाह देने, जैसा उपयु त हो, के िलए उ त अिधिनयम क3 धारा 9 क3 उप धारा (1) के अंतगत शD तयां ूदान क3 गई हS; और जबAक, उ त के ि/ य सूची को एनसीबीसी क3 िसफा@रशU पर संशोिधत Aकया गया था और अनुबंधअनुबंध----II मQ यथािनAदं ट संक प के तहत के ि/ य सरकार ारा समय -समय पर अिधसूिचत Aकया गया था ; 640 GI/2014 (1) 2 THE GAZETTE OF INDIA : EXTRAORDINARY [P ART I—SEC . -
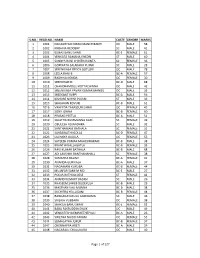
S.No. Regd.No. Name Caste Gender Marks 1 1001
S.NO. REGD.NO. NAME CASTE GENDER MARKS 1 1001 NAGASHYAM KIRAN MANCHIKANTI OC MALE 58 2 1002 KRISHNA REDDERY SC MALE 41 3 1003 ELMAS BANU SHAIK BC-E FEMALE 61 4 1004 VENKATA RAMANA KHEDRI ST MALE 60 5 1005 SANDYA RANI CHINTHAKUNTA SC FEMALE 36 6 1006 GOPINATH SALAKARU PUJARI SC MALE 28 7 1007 SREENIVASA REDDY GOTLURI OC MALE 78 8 1008 LEELA RANI B BC-A FEMALE 57 9 1009 RADHIKA KONDA OC FEMALE 30 10 1010 SREEDHAR M BC-D MALE 68 11 1011 CHANDRAMOULI KOTTACHINNA OC MALE 42 12 1012 SREENIVASA PAVAN KUMAR MANGU OC MALE 35 13 1013 SREEKANT SUPPI BC-A MALE 56 14 1014 KISHORE NAYAK PUJARI ST MALE 39 15 1015 SHAJAHAN KOVURI BC-B MALE 61 16 1016 VAHEEDA TABASSUM SHAIK OC FEMALE 45 17 1017 SONY JONNA BC-B FEMALE 60 18 1018 PRASAD PEETLA BC-A MALE 51 19 1019 SUJATHA BUMMANNA GARI SC FEMALE 49 20 1020 OBULESH ADIANDHRA SC MALE 32 21 1021 SANTHAMANI BATHALA SC FEMALE 31 22 1022 SARASWATHI GOLLA BC-D FEMALE 47 23 1023 LAVANYA GAJULA OC FEMALE 55 24 1024 SATEESH KUMAR MAHESWARAM BC-B MALE 38 25 1025 KRANTHI NALLAGATLA BC-B FEMALE 33 26 1026 RAVI KUMAR BATHALA BC-B MALE 68 27 1027 ADI LAKSHMI BANTHANAHALL SC FEMALE 38 28 1028 SAMATHA BALIMI BC-A FEMALE 41 29 1030 ANANDA GURIKALA BC-A MALE 37 30 1031 NAGAMANI KURUBA BC-B FEMALE 44 31 1032 MUJAFAR SAMI M MD BC-E MALE 27 32 1033 POOJA RATHOD DESE ST FEMALE 42 33 1034 ANAND KUMART BADIGI SC MALE 26 34 1035 KHASEEM SAHEB DUDEKULA BC-B MALE 29 35 1036 MASTHAN VALI MUNNA BC-B MALE 38 36 1037 SUCHITRA YELLUGANI BC-B FEMALE 44 37 1038 RANGANAYAKULU GUDIDAMA SC MALE 46 38 1039 SAILAJA VUBBARA OC FEMALE 38 39 1040 SHAKILA BANU SHAIK BC-E FEMALE 52 40 1041 BABA FAKRUDDIN SHAIK OC MALE 49 41 1042 VENKATESH DEMAKETHEPALLI BC-A MALE 26 42 1043 SWETHA NAIDU PAKAM OC FEMALE 55 43 1044 SUMALATHA JUKUR BC-B FEMALE 37 44 1047 CHENNAPPA ARETI BC-A MALE 29 45 1048 NAGARAJU CHALUKURU OC MALE 40 Page 1 of 127 S.NO. -
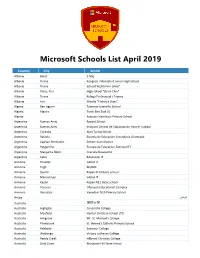
Microsoft Schools List April 2019
Microsoft Schools List April 2019 Country City School Albania Berat 5 Maj Albania Tirane Kongresi i Manastirit Junior High School Albania Tirane School"Kushtrimi i Lirise" Albania Patos, Fier High school "Zhani Ciko" Albania Tirana Kolegji Profesional i Tiranës Albania Fier Shkolla "Flatrat e Dijes" Algeria Ben Isguen Tawenza Scientific School Algeria Algiers Tarek Ben Ziad 01 Algeria Azzoune Hamlaoui Primary School Argentina Buenos Aires Bayard School Argentina Buenos Aires Instituto Central de Capacitación Para el Trabajo Argentina Cordoba Alan Turing School Argentina Rafaela Escuela de Educación Secundaria Orientada Argentina Capitan Bermudez Doctor Juan Alvarez Argentina Pergamino Escuela de Educacion Tecnica N°1 Argentina Margarita Belen Graciela Garavento Argentina Caba Educacion IT Armenia Hrazdan Global It Armenia Tegh MyBOX Armenia Syunik Kapan N 13 basic school Armenia Mikroshrjan Global IT Armenia Kapan Kapan N13 basic school Armenia Yerevan Ohanyan Educational Complex Armenia Vanadzor Vanadzor N19 Primary School الرياض Aruba Australia 坦夻易锡 Australia Highgate Concordia College Australia Mayfield Hunter Christian School LTD Australia Ashgrove Mt. St. Michael’s College Australia Ellenbrook St. Helena's Catholic Primary School Australia Adelaide Seymour College Australia Wodonga Victory Lutheran College Australia Reedy Creek Hillcrest Christian College Australia Gold Coast Musgrave Hill State School Microsoft Schools List April 2019 Country City School Australia Plainland Faith Lutheran College Australia Beaumaris Beaumaris North Primary School Australia Roxburgh Park Roxburgh Park Primary School Australia Mooloolaba Mountain Creek State High School Australia Kalamunda Kalamunda Senior High School Australia Tuggerah St. Peter's Catholic College, Tuggerah Lakes Australia Berwick Nossal High School Australia Noarlunga Downs Cardijn College Australia Ocean Grove Bellarine Secondary College Australia Carlingford Cumberland High School Australia Thornlie Thornlie Senior High School Australia Maryborough St.