20010048006.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Engineering Behavior and Classification of Lateritic Soils in Relation to Soil Genesis Erdil Riza Tuncer Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1976 Engineering behavior and classification of lateritic soils in relation to soil genesis Erdil Riza Tuncer Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Civil Engineering Commons Recommended Citation Tuncer, Erdil Riza, "Engineering behavior and classification of lateritic soils in relation to soil genesis " (1976). Retrospective Theses and Dissertations. 5712. https://lib.dr.iastate.edu/rtd/5712 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Topic: Soil Classification
Programme: M.Sc.(Environmental Science) Course: Soil Science Semester: IV Code: MSESC4007E04 Topic: Soil Classification Prof. Umesh Kumar Singh Department of Environmental Science School of Earth, Environmental and Biological Sciences Central University of South Bihar, Gaya Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data/figures/materials are taken from several research articles/e-books/text books including Wikipedia and other online resources. 1 • Pedology: The origin of the soil , its classification, and its description are examined in pedology (pedon-soil or earth in greek). Pedology is the study of the soil as a natural body and does not focus primarily on the soil’s immediate practical use. A pedologist studies, examines, and classifies soils as they occur in their natural environment. • Edaphology (concerned with the influence of soils on living things, particularly plants ) is the study of soil from the stand point of higher plants. Edaphologist considers the various properties of soil in relation to plant production. • Soil Profile: specific series of layers of soil called soil horizons from soil surface down to the unaltered parent material. 2 • By area Soil – can be small or few hectares. • Smallest representative unit – k.a. Pedon • Polypedon • Bordered by its side by the vertical section of soil …the soil profile. • Soil profile – characterize the pedon. So it defines the soil. • Horizon tell- soil properties- colour, texture, structure, permeability, drainage, bio-activity etc. • 6 groups of horizons k.a. master horizons. O,A,E,B,C &R. 3 Soil Sampling and Mapping Units 4 Typical soil profile 5 O • OM deposits (decomposed, partially decomposed) • Lie above mineral horizon • Histic epipedon (Histos Gr. -

Subsurface Stratigraphy and Genesis of Pre-Wisconsin Paleosols in Whitebreast Creek Watershed, South-Central Iowa
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1995 Subsurface stratigraphy and genesis of pre- Wisconsin paleosols in Whitebreast Creek Watershed, south-central Iowa Amir Hossein Charkhabi Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agricultural Science Commons, Agriculture Commons, Agronomy and Crop Sciences Commons, Geology Commons, and the Mineral Physics Commons Recommended Citation Charkhabi, Amir Hossein, "Subsurface stratigraphy and genesis of pre-Wisconsin paleosols in Whitebreast Creek Watershed, south- central Iowa " (1995). Retrospective Theses and Dissertations. 10887. https://lib.dr.iastate.edu/rtd/10887 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfihn master. UMI filmc the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in ^pewriter face, while others may be from any type of computer printer. The qnalify of this reprodnction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and inqjroper aligmnent can adversefy affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

Redalyc.CONTAMINATION POTENTIAL of SPECIFIC IONS IN
Revista Caatinga ISSN: 0100-316X [email protected] Universidade Federal Rural do Semi- Árido Brasil PAIVA DE OLIVEIRA, ANDLER MILTON; MEDEIROS REBOUÇAS, CEZAR AUGUSTO; DA SILVA DIAS, NILDO; CRUZ PORTELA, JEANE; ARAÚJO DINIZ, ADRIANA CONTAMINATION POTENTIAL OF SPECIFIC IONS IN SOIL TREATED WITH REJECT BRINE FROM DESALINATION PLANTS Revista Caatinga, vol. 29, núm. 3, julio-septiembre, 2016, pp. 569-577 Universidade Federal Rural do Semi-Árido Mossoró, Brasil Available in: http://www.redalyc.org/articulo.oa?id=237146823005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Universidade Federal Rural do Semi-Árido ISSN 0100-316X (impresso) Pró-Reitoria de Pesquisa e Pós-Graduação ISSN 1983-2125 (online) http://periodicos.ufersa.edu.br/index.php/sistema CONTAMINATION POTENTIAL OF SPECIFIC IONS IN SOIL TREATED WITH REJECT BRINE FROM DESALINATION PLANTS1 ANDLER MILTON PAIVA DE OLIVEIRA2, CEZAR AUGUSTO MEDEIROS REBOUÇAS2, NILDO DA SILVA DIAS2*, JEANE CRUZ PORTELA2, ADRIANA ARAÚJO DINIZ2 ABSTRACT - Percolation columns constructed in the Laboratory can predict the degree of contamination in soil due to reject brine disposal and can be a tool for reducing environmental impacts. This study aim to evaluate the mobilization of ions in reject brine from desalination process by reverse osmosis. The mobilization of the contaminant ions in the saline waste was studied in glass percolation columns, which were filled with soil of contrasting textures (eutrophic CAMBISOL, typic dystrophic Red OXISOL, ENTISOL Quartzipsamment). -

One Year Residual Effect of Sewage Sludge Biochar As a Soil Amendment for Maize in a Brazilian Oxisol
sustainability Article One Year Residual Effect of Sewage Sludge Biochar as a Soil Amendment for Maize in a Brazilian Oxisol Joisman Fachini 1 , Thais Rodrigues Coser 1 , Alyson Silva de Araujo 1 , Ailton Teixeira do Vale 2, Keiji Jindo 3 and Cícero Célio de Figueiredo 1,* 1 Faculty of Agronomy and Veterinary Medicine, University of Brasilia, Brasília 70910970, Brazil; [email protected] (J.F.); [email protected] (T.R.C.); [email protected] (A.S.d.A.) 2 Department of Forestry Engineering, University of Brasilia, Brasília 70910970, Brazil; [email protected] 3 Agrosystems Research, Wageningen University & Research, P.O. Box 16, 6700 AA Wageningen, The Netherlands; [email protected] * Correspondence: [email protected]; Tel.: +55-61-3107-7564 Abstract: The thermochemical transformation of sewage sludge (SS) to biochar (SSB) allows exploring the advantages of SS and reduces possible environmental risks associated with its use. Recent studies have shown that SSB is nutrient-rich and may replace mineral fertilizers. However, there are still some questions to be answered about the residual effect of SSB on soil nutrient availability. In addition, most of the previous studies were conducted in pots or soil incubations. Therefore, the residual effect of SSB on soil properties in field conditions remains unclear. This study shows the results of nutrient availability and uptake as well as maize yield the third cropping of a three-year consecutive corn cropping system. The following treatments were compared: (1) control: without mineral fertilizer and biochar; (2) NPK: with mineral fertilizer; (3) SSB300: with biochar produced at ◦ ◦ 300 C; (4) SSB300+NPK; (5) SSB500: with biochar produced at 500 C; and (6) SSB500+NPK. -
Liming in the Tropics: Variable-Charge Soils May Be Highly Buffered
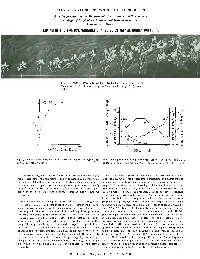
ILLUSTRATED CONCEPTS IN TROPICAL AGRICULTURE A series prepared by the Department of Agronomy and Soil Science College of Tropical Agriculture and Human Resources University of Hawaii LIMING IN THE TROPICS: VARIABLE-CHARGE SOILS MAY BE HIGHLY BUFFERED Fig. I. Growth of cowpea across a pH gradient produced in an Oxisol (Wahiawa series) by liming from pH 4.7 (extreme left) to pH 7.1 (extreme right). 7 OXISOL ,.------- 0. ; a /' Q / , I c:r E , ...... / Q) ,.4 c:r .08E, 01 Q) 6 / S 01 / r- 3- S ~ / <..> I od: z z Z Q. 0::1.0 0 \M"/ 0 / , . 0 I r- ~ • X :::>.2 • 4~ .04i= 5 I W -...l /' :::> I '" \ c5 -...l 0.5 @ \ en ". en0 ::lr::: ~ -- . ~ z c: Z AI"'..:-"" ...... - ..!':"---o 0 4'------'-----1.-----' ~ .0 ~.O <..> .0 a.. o 10 20 30 5 6 7 CaC0 (meq/IOOg) 3 SOIL pH Fig. 2. Lime curve produced by adding increasing quantities of CaCO to Fig. 3. Changes in the solubility or extractability of A I. Mn, P and Ca in an Oxisol (Wahiawa series). 3 relation to the pH to which an Oxisol (Wahiawa series) had been limed. Some tropicallegumes-·cowpea for example-are generally believed to Important soil management concepts come from these relationships: (I) require less lime than temperate legumes require. Such comparisons At low soil pH. Oxisols require less lime to precipitate toxic aluminum (and may not be valid. although an important distinction may be made between perhaps manganese too) than do constant charge soils in the same pH the soils on which tropical legumes and temperate legumes most frequently range. -

Supplementary Information
Supplementary information: SOC Stock Changes and Greenhouse Gas Emissions Following Tropical Land Use Conversions to Plantation Crops on Mineral Soils with a Special Focus on Oil Palm and Rubber Plantations Sanjutha Shanmugam 1,*, Ram C. Dalal 1, Hans Joosten 2, R J Raison 3 and Goh Kah Joo 4 Limitations of the dataset Most of the studies referred to in this review followed paired-site sampling technique; the relative changes in SOC stock and GHG emissions with reference to land use conversion are mostly estimated assuming linear changes over time (between 1-35 years). Although the paired-site sampling is largely used as an appropriate tool for ecological and soil development studies (Walker et al., 2010), the paired-site approach is less likely to be adaptable when there are higher disturbances and unpredictable changes occur in soil. Hence, there is a potential risk in relying on the paired-site sampling alone in predicting SOC stock and GHG emissions and applied for land use conversions (HCS, 2015). For example, soil sampling depth acts as a critical unknown factor that affects C storage and SOC stock measurement in oil palm plantations. IPCC (2006) proposes default sampling of the upper 30 cm of soil, whereas various studies in oil palm plantations have collected samples at a range of sampling depths (e.g., increments of 10-15 cm up to 50 cm or increments of 50 cm down to 150-300 cm; Ng et al., 2011; Padmanabhan et al., 2013; van Straaten et al., 2015). Such an extended sampling depth takes the deeper root zone into account and arrives at substantial SOC under oil palm. -

Characterization of Yellow Latosols (Oxisols) of Serra Do Quilombo, in Piauí State Savanna Woodlands - Brazil1
Universidade Federal Rural do Semi-Árido ISSN 0100-316X (impresso) Pró-Reitoria de Pesquisa e Pós-Graduação ISSN 1983-2125 (online) http://periodicos.ufersa.edu.br/index.php/sistema http://dx.doi.org/10.1590/1983-21252016v29n407rc CHARACTERIZATION OF YELLOW LATOSOLS (OXISOLS) OF SERRA DO QUILOMBO, IN PIAUÍ STATE SAVANNA WOODLANDS - BRAZIL1 ROSSANNA BARBOSA PRAGANA2*, VALDOMIRO SEVERINO DE SOUZA JUNIOR3, REGIANA DOS SANTOS MOURA4, JORDÂNIA MEDEIROS SOARES5 ABSTRACT – The savanna woodlands of Piauí state has great economic importance since it is an area for agricultural expansion, being the fourth most important of Brazil and the first from Brazilian Northeastern. The area accounts for 5.9% of the Brazilian savanna woodlands and 36.9% of the Northeastern savanna, covering 46% of the Piauí state area, in a total of 11.5 million hectares. The goal of this research was to study pedoenvironments of Serra do Quilombo region, which is in Piauí state savanna, as well as identifying existing soil classes, according to the Brazilian System of Soil Classification - SiBCS. Soil identification consisted in characterizing soil profiles along a transect, assessing in-field conditions and collecting soil samples, in areas of native vegetation. The samples were gathered from three distinct points, being two at the edges and one at the center of the plateau. Soil analyses were carried out with samples collected from each horizon through trench digging up to a 2-m depth. Morphological, physical, chemical and mineralogical characterizations were performed for each soil profile, along with an evaluation of the effect of pedogenic factors on their formation and development. All soils under study were formed with source materials of the same geological formation; however, each rock has a distinct contribution to the process, involving sandstones and shales. -

Characterization and Classification of Some Soils of Hawaii with Andic and Oxic Properties a Thesis Submitted to the Graduate Di
CHARACTERIZATION AND CLASSIFICATION OF SOME SOILS OF HAWAII WITH ANDIC AND OXIC PROPERTIES A THESIS SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAII IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN SOIL SCIENCE DECEMBER, 1990 By Nicole Simmons Thesis Committee: Haruyoshi Ikawa, Chairperson James A. Silva Christopher W. Smith We certify that we have read this thesis and that, in our opinion, it is satisfactory in scope and quality as a thesis for the degree of Masters in Science in Soil Science. THESIS COMMITTEE Chairperson ii ACKNOWLEDGEMENTS I wish to thank Dr. Robert T. Gavenda for suggesting the topic for this study. I also thank the staff of the State Office of the Soil Conservation Service for their continual support and encouragement. Ill TABLE OF CONTENTS CHAPTER 1. INTRODUCTION ................................. 1 CHAPTER 2. REVIEW OF LITERATURE ........................ 6 Andlsols ............................................ 6 Central C9 Pg?ptg of ttig Or<aer__. 6 Definition of Andisols ........................ 9 ggle&tg<^ physigal Properties gf AndigPls . .13 Chemical Properties of A n d i s o l s .............. 16 O x i s o l s ............................................... 21 Central Concepts og tUe Qxis<?l Qr<a? r ......... 2 1 Definition of Oxis o l s ......................... 26 The oxic horizon ................................28 The Andisol-Oxisol Transition ....................... 36 CHAPTER 3. MATERIALS AND M E T H O D S .......................40 The Soil Series ...................................... 40 The Laboratory D a t a .................................. 4 3 The m Value ...........................................44 The Taxonomic Classification of Soils .............. 45 CHAPTER 4. RESULTS AND DISCUSSION ....................... 46 The Soils and Their Properties .....................46 The m Values and Andic-Oxic S oils ................. -

Agricultural Management of an Oxisol Affects Accumulation of Heavy Metals
Chemosphere 185 (2017) 344e350 Contents lists available at ScienceDirect Chemosphere journal homepage: www.elsevier.com/locate/chemosphere Agricultural management of an Oxisol affects accumulation of heavy metals Guilherme Deomedesse Minari a, David Luciano Rosalen b, Mara Cristina Pessoa^ da Cruz c, * Wanderley Jose de Melo a, d, Lucia Maria Carareto Alves a, Luciana Maria Saran a, a Technology Department, Sao~ Paulo State University (UNESP), School of Agricultural and Veterinarian Sciences, Acesso Prof. Paulo Donato Castellane, S/N, 14884-900, Jaboticabal, SP, Brazil b Rural Engineering Department, Sao~ Paulo State University (UNESP), School of Agricultural and Veterinarian Sciences, Acesso Prof. Paulo Donato Castellane, S/N, 14884-900, Jaboticabal, SP, Brazil c Soils and Fertilizers Department, Sao~ Paulo State University (UNESP), School of Agricultural and Veterinarian Sciences, Acesso Prof. Paulo Donato Castellane, S/N, 14884-900, Jaboticabal, SP, Brazil d Universidade Brasil, Av. Hilario da Silva Passos, 950, 13690-000, Descalvado, SP, Brazil highlights Cd, Cr and Ni levels in the native forest exceeded the reference quality values. 12% of the experimental area is contaminated with Cd, 16% with Cr and 0.3% with Ni. The management with sewage sludge increased the levels of Cd and Ni. The Cr content in soil was not affected by the type of management. article info abstract Article history: Soil contamination may result from the inadequate disposal of substances with polluting potential or Received 20 December 2016 prolonged agricultural use. Therefore, cadmium (Cd), chromium (Cr) and nickel (Ni) concentrations were Received in revised form assessed in a Eutroferric Red Oxisol under a no-tillage farming system with mineral fertilizer applica- 26 May 2017 tions, a conventional tillage system with mineral fertilizer application and a conventional tillage system Accepted 2 July 2017 with sewage sludge application in an area used for agriculture for more than 80 years. -

Weathering and Development of Chemically Mature Soils - Nicolas Fedoroff
EARTH SYSTEM: HISTORY AND NATURAL VARIABILITY - Vol. II - Weathering and Development of Chemically Mature Soils - Nicolas Fedoroff WEATHERING AND DEVELOPMENT OF CHEMICALLY MATURE SOILS Nicolas Fedoroff Institut National Agronomique, Thiverval-Grignon, France Keywords: bauxite, ferricrete (iron crust), Gondwanean cratons, hydrolysis, laterite, Oxisol (Ferrasol), pedoplasmation, stone line, Ultisol (Acrisol), weathering. Contents 1. Introduction 2. Morphology and Classification of Chemically Mature Soils 2.1. Alterites 2.2. Chemically Mature Soils: Diagnostic Horizons and Characters 3. The Processes of Weathering of Igneous, Metamorphic, and Volcanic Rocks 3.1. Principles 3.2. Primary Mineral Weathering 3.3. Stages of Weathering 4. Pedoplasmation 4.1. Processes of Pedoplasmation 5. Sesquioxide Aggradation and Degradation 5.1. Principles 5.2. Morphology of Ferruginous Nodules 5.3. Cyclic Evolution of Complex Sesquioxidic Concentrations 5.4. Morphology and Genesis of Iron Crusts (Ferricretes) 6. Genesis of Subsurface Diagnostic Horizons in Chemically Mature Soils 6.1. Oxic Horizon 6.2. Argillic Horizons 7. Role of Erosion and Sedimentation in the Development of Chemically Mature Soils; Application to Landform Evolution on Cratons; Examples from West African Cratons 7.1. Role of Sesquioxidic Concentrations in Landform Development and Distribution 7.2. Behavior of Oxic Materials through Time 7.3. Significance of Stone Lines 7.4. Pleistocene and Holocene Landscape Evolution in Western Africa 8. ChemicallyUNESCO Mature Soils: Past and Present – LanduseEOLSS 8.1. Constraints for Modern Agriculture of Chemically Mature and Derived Soils 8.2. Human-induced Degradation of Chemically Mature and Derived Soils 8.3. SustainableSAMPLE Agriculture on Chemically Mature CHAPTERS and Derived Soils 9. Conclusions Glossary Bibliography Biographical Sketch Summary Chemically mature soils are the result of a long and complex genesis. -

Chapter 3 Chapter 3 Learning Objectives
Chapter 3 Learning Objectives Chapter 3 • Describe the current USDA soil classification system • List the six categories of classification in Soil Soil Taxonomy Taxonomy • Describe the major characteristics, the general degree of weathering and soil development, and the worldwide distribution and uses of the 12 soil orders • List key features of a particular soil and its environment given the soil name (e.g., Hapludalf) Diagnostic Subsurface Horizons Diagnostic Subsurface Horizons • 18 of them – Albic: light-colored elluvial horizon (leached) – Cambic: weakly developed horizon, some color • Six we will focus on (and the assoc. genetic change label): – Spodic: illuvial horizon with accumulations of O.M. – Argillic: subsurface accumulations of silicate clays – Albic (E) -Argg(illic (Bt) – CliCalcic: accumu ltilation o f car bona tes, o ften as – Cambic (Bw) - Fragic (Bx) white, chalk-like nodules – Spodic (Bhs) - Calcic (Bk) – Fragipan: cemented, dense, brittle pan Light colored horizon Albic Argillic Weakly developed horizon Cambic No significant accumulation silicate clays Unweathered Accumulation of Acid weathering, material organic matter Fe, Al oxides Spodic Calcic Fragipan Modified from full version of Figure 3.3 in textbook (page 62). 1 Levels of Description Levels of Description • Order Most general • Order – One name, all end in “-sol” There are 12. Differentiated by presence or absence of • Suborder diagnostic horizons or features that reflect • Great group soil-forming processes. EXAMPLE: ENTISOL • Subgroup • Suborder • Family • Great group • Subgroup •Series •Family Most specific •Series Levels of Description Levels of Description • Order – One name, all end in “-sol” There are 12 . • Order – One name, all end in “-sol” There are 12 .