In-Depth Characterization of the Wnt-Signaling/Β-Catenin Pathway In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

G Protein‐Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology (2019) 176, S21–S141 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors Stephen PH Alexander1 , Arthur Christopoulos2 , Anthony P Davenport3 , Eamonn Kelly4, Alistair Mathie5 , John A Peters6 , Emma L Veale5 ,JaneFArmstrong7 , Elena Faccenda7 ,SimonDHarding7 ,AdamJPawson7 , Joanna L Sharman7 , Christopher Southan7 , Jamie A Davies7 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Monash Institute of Pharmaceutical Sciences and Department of Pharmacology, Monash University, Parkville, Victoria 3052, Australia 3Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK 4School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 5Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 6Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 7Centre for Discovery Brain Sciences, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. Although the Concise Guide represents approximately 400 pages, the material presented is substantially reduced compared to information and links presented on the website. -

Wnt/Rspondin/Β-Catenin Signals Control Axonal Sorting and Lineage Progression in Schwann Cell Development
Wnt/Rspondin/β-catenin signals control axonal sorting and lineage progression in Schwann cell development Tamara Grigoryana, Simone Steina, Jingjing Qia, Hagen Wendeb, Alistair N. Garrattc, Klaus-Armin Naved, Carmen Birchmeierb, and Walter Birchmeiera,1 aCancer Research Program and bNeuroscience Program, Max Delbrück Center for Molecular Medicine, 13125 Berlin, Germany; cCenter for Anatomy, Charité University Hospital, 10117 Berlin, Germany; and dDepartment of Neurogenetics, Max Planck Institute for Experimental Medicine, 37075 Göttingen, Germany Edited by Thomas C. Südhof, Stanford University School of Medicine, Stanford, CA, and approved September 26, 2013 (received for review June 2, 2013) During late Schwann cell development, immature Schwann cells organs (24–27). A role of Rspondins and Lgr4–6 receptors in SC segregate large axons from bundles, a process called “axonal ra- development has not been studied. dial sorting.” Here we demonstrate that canonical Wnt signals play Here we define a temporal window of Wnt/β-catenin activity a critical role in radial sorting and assign a role to Wnt and Rspon- and its role in SC lineage progression using mouse genetics and din ligands in this process. Mice carrying β-catenin loss-of-function cell culture techniques. Conditional loss-of-function (LOF) and mutations show a delay in axonal sorting; conversely, gain-of-function gain-of-function (GOF) mutations of β-catenin in mouse SCs mutations result in accelerated sorting. Sorting deficits are accom- produce converse phenotypes: a delay and an acceleration of panied by abnormal process extension, differentiation, and aber- axonal sorting, respectively. Using cultured primary SCs and rant cell cycle exit of the Schwann cells. -

FZD2 Inhibits the Cell Growth and Migration of Salivary Adenoid Cystic Carcinomas
1006 ONCOLOGY REPORTS 35: 1006-1012, 2016 FZD2 inhibits the cell growth and migration of salivary adenoid cystic carcinomas LIN-CAN DING1*, XIAO-YU HUANG1*, FEI-FEI ZHENG1*, JIAN XIE1, LIN SHE1, YAN FENG1, BO-HUA SU1, DA-LI ZHENG2 and YOU-GUANG LU1 1Department of Preventive Dentistry, Affiliated Stomatological Hospital, Fujian Medical University, Fuzhou 350002; 2Key Laboratory of Ministry of Education for Gastrointestinal Cancer, School of Basic Medical Sciences, Fujian Medical University, Fuzhou 350108, P.R. China Received November 14, 2014; Accepted October 24, 2015 DOI: 10.3892/or.2015.3811 Abstract. Several studies have reported that FZD2 regulates Introduction tumor biology in a complex manner. The aim of the present study was to identify the role of FZD2 in the cell growth and Salivary adenoid cystic carcinomas (SACCs) are malignant metastasis of salivary adenoid cystic carcinomas (SACCs). tumors of the head and neck and are characterized by unique The expression of FZD2 in ACC-83 and ACC-LM cells were clinical features and behaviors. SACCs occur in the major and measured with real-time PCR. Immunohistochemical staining minor salivary glands and disperse to the oral and oropharyn- was used to detect the expression of FZD2 in clinical SACC geal mucosa, tracheobronchial tree and the esophagus. The samples with or without metastasis. Cell proliferation and biological properties of this dispersal include slow and indolent Transwell assays were performed to explore the effects of FZD2 growth, a low probability of regional nodal metastasis, a high on cell growth and migration following the silencing of FZD2 propensity for perineural migration and distant metastasis, with small interference RNAs and the overexpression of FZD2 and a high incidence of recurrence. -

Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells Amy Sue Bogard University of Tennessee Health Science Center
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 12-2013 Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells Amy Sue Bogard University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Medical Cell Biology Commons, and the Medical Molecular Biology Commons Recommended Citation Bogard, Amy Sue , "Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells" (2013). Theses and Dissertations (ETD). Paper 330. http://dx.doi.org/10.21007/etd.cghs.2013.0029. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells Document Type Dissertation Degree Name Doctor of Philosophy (PhD) Program Biomedical Sciences Track Molecular Therapeutics and Cell Signaling Research Advisor Rennolds Ostrom, Ph.D. Committee Elizabeth Fitzpatrick, Ph.D. Edwards Park, Ph.D. Steven Tavalin, Ph.D. Christopher Waters, Ph.D. DOI 10.21007/etd.cghs.2013.0029 Comments Six month embargo expired June 2014 This dissertation is available at UTHSC Digital Commons: https://dc.uthsc.edu/dissertations/330 Adenylyl Cyclase 2 Selectively Regulates IL-6 Expression in Human Bronchial Smooth Muscle Cells A Dissertation Presented for The Graduate Studies Council The University of Tennessee Health Science Center In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy From The University of Tennessee By Amy Sue Bogard December 2013 Copyright © 2013 by Amy Sue Bogard. -

Supplemental Material
Supplemental material Systems Pharmacology Approach to Prevent Retinal Degeneration in Stargardt Disease Yu Chen, Grazyna Palczewska, Debarshi Mustafi, Marcin Golczak, Zhiqian Dong, Osamu Sawada, Tadao Maeda, Akiko Maeda, and Krzysztof Palczewski Table of Contents: 1. Supplemental Table 1. 2. Supplemental Table 2. 3. Supplemental Table 3. 4. References. 1 Supplemental Table 1. Expression of GPCRs in the eye and retina of C57BL/6J mice and the retina of a human donor eye (normalized FPKM values)A. Genes B6 mouse B6 mouse Human retina eye retina Rho 6162.04 11630.18 6896.09 Rgr 355.74 97.66 123.98 Opn1sw 125.13 198.54 31.69 Drd4 93.84 241.78 139.49 Opn1mw 62.97 95.77 172.56 Gprc5b 29.82 12.95 22.85 Gpr162 29.37 73.32 46.29 Gpr37 28.47 41.28 66.65 Ednrb 22.27 1.94 5.77 Rorb 21.69 23.52 24.31 Gpr153 20.42 37.18 15.31 Gabbr1 19.78 40.24 35.38 Rrh 19.29 9.23 40.34 2 Gpr152 18.55 40.46 3.05 Adora1 16.20 18.26 13.55 Lphn1 15.98 29.73 31.85 Tm2d1 15.56 10.31 17.63 Cxcr7 14.30 3.58 2.37 Ppard 13.68 19.37 21.61 Agtrap 13.64 17.21 8.18 Cd97 12.93 1.77 1.55 Gpr19 12.21 8.45 1.11 Fzd1 11.99 3.29 7.35 Fzd6 11.34 1.85 2.76 Gpr87 11.34 0.04 0.00 Lgr4 11.09 9.50 18.07 Drd2 10.82 23.10 26.33 Smo 10.75 6.35 5.91 S1pr1 10.66 11.21 11.78 Bai1 10.08 27.10 10.82 3 Glp2r 9.94 34.85 0.31 Ptger1 9.59 14.88 0.94 Gpr124 9.56 8.94 19.82 F2r 9.31 5.32 0.15 Adra2c 8.96 7.17 2.38 Gpr146 8.91 7.49 6.17 Vipr2 8.79 14.33 10.69 Fzd5 8.69 10.01 7.73 Gpr110 8.59 0.08 0.02 Adrb1 8.43 20.18 3.84 S1pr3 8.42 6.95 3.56 Gabbr2 7.80 17.03 10.57 Lphn2 7.66 9.02 8.79 Lpar1 7.47 0.91 -

Supplementary Materials for Volumetric Alteration of Olfactory
Supplementary materials for Volumetric alteration of olfactory bulb and immune-related molecular changes in olfactory epithelium in first episode psychosis patients Kun Yang1,#, Jun Hua2,7,#, Semra Etyemez1, Adrian Paez7, Neal Prasad1, Koko Ishizuka1, Akira Sawa1,2,3,4,5,6,#,*, and Vidyulata Kamath1,# Departments of Psychiatry1, Radiology and RadiologiCal SCiences2, NeurosCience3, BiomediCal Engineering4, and GenetiC MediCine5, Johns Hopkins University SChool of MediCine, Baltimore, Maryland. Department of Mental Health6, Johns Hopkins Bloomberg SChool of PubliC Health, Baltimore, Maryland. F.M. Kirby Research Center for Functional Brain Imaging7, Kennedy Krieger Institute, Baltimore, Maryland. # These authors contributed equally to this work. * Corresponding and contaCt author: Akira Sawa, [email protected] Table S1. Immune-related disorders for the GWAS enrichment analysis. Traits acquired immunodeficiency syndrome (AIDS) allergic rhinitis allergy amyloid light-chain (AL) amyloidosis asthma atopic eczema B-cell acute lymphoblastic leukemia cryoglobulinemia dengue Hemorrhagic Fever dilated cardiomyopathy duodenal ulcer hepatic fibrosis hepatitis hepatitis C induced liver cirrhosis HIV-1 infection hodgkins lymphoma idiopathic pulmonary fibrosis immune system disease inflammatory bowel disease influenza A (H1N1) lymphoma malaria marginal zone B-cell lymphoma mixed cellularity monoclonal gammopathy multiple myeloma myositis osteitis deformans osteoarthritis pancreatitis periodontitis psoriasis psoriasis vulgaris psoriatic arthritis recalcitrant -

Frizzled7: a Promising Achilles' Heel for Targeting the Wnt Receptor
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Online Research @ Cardiff cancers Review Frizzled7: A Promising Achilles’ Heel for Targeting the Wnt Receptor Complex to Treat Cancer Toby Phesse 1,2,*,†,‡, Dustin Flanagan 1,† and Elizabeth Vincan 1,3,* 1 Molecular Oncology Laboratory, Victorian Infectious Diseases Reference Laboratory and the Doherty Institute, University of Melbourne, Melbourne, VIC 3000, Australia; dustin.fl[email protected] 2 Walter and Eliza Hall Institute of Medical Research, Melbourne, VIC 3052, Australia 3 School of Biomedical Sciences, Curtin University, Perth, WA 6102, Australia * Correspondence: [email protected] (T.P.); [email protected] (E.V.); Tel.: +44-29-2087-4089 (T.P.); +61-(0)-9342-9348 (E.V.) † These authors contributed equally to this work. ‡ Current Address: European Cancer Stem Cell Research Institute, Cardiff University, Cardiff, CF24 4HQ, UK. Academic Editors: Renée van Amerongen and Walter Birchmeier Received: 31 March 2016; Accepted: 9 May 2016; Published: 17 May 2016 Abstract: Frizzled7 is arguably the most studied member of the Frizzled family, which are the cognate Wnt receptors. Frizzled7 is highly conserved through evolution, from Hydra through to humans, and is expressed in diverse organisms, tissues and human disease contexts. Frizzled receptors can homo- or hetero-polymerise and associate with several co-receptors to transmit Wnt signalling. Notably, Frizzled7 can transmit signalling via multiple Wnt transduction pathways and bind to several different Wnt ligands, Frizzled receptors and co-receptors. These promiscuous binding and functional properties are thought to underlie the pivotal role Frizzled7 plays in embryonic developmental and stem cell function. -

Maternal Low-Fat Diet Programs the Hepatic Epigenome Despite Exposure to an Obesogenic Postnatal Diet
nutrients Article Maternal Low-Fat Diet Programs the Hepatic Epigenome despite Exposure to an Obesogenic Postnatal Diet Laura Moody 1, Justin Shao 2, Hong Chen 3 and Yuan-Xiang Pan 4,* 1 Division of Nutritional Sciences, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA 2 Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Exeter High School, 1 Blue Hawk Drive, Exeter, NH 03833, USA 3 Department of Food Science and Human Nutrition, Division of Nutritional Sciences, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA 4 Department of Food Science and Human Nutrition, Division of Nutritional Sciences, and Illinois Informatics Institute, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA * Correspondence: [email protected]; Tel.: +1-217-333-3466 Received: 29 June 2019; Accepted: 27 August 2019; Published: 3 September 2019 Abstract: Obesity and metabolic disease present a danger to long-term health outcomes. It has been hypothesized that epigenetic marks established during early life might program individuals and have either beneficial or harmful consequences later in life. In the present study, we examined whether maternal diet alters DNA methylation and whether such modifications persist after an obesogenic postnatal dietary challenge. During gestation and lactation, male Sprague-Dawley rats were exposed to either a high-fat diet (HF; n = 10) or low-fat diet (LF; n = 10). After weaning, all animals were fed a HF diet for an additional nine weeks. There were no differences observed in food intake or body weight between groups. Hepatic DNA methylation was quantified using both methylated DNA immunoprecipitation sequencing (MeDIP-seq) and methylation-sensitive restriction enzyme sequencing (MRE-seq). -
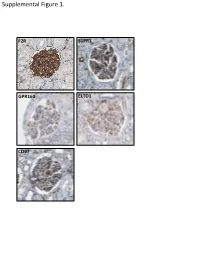
Supplemental Data
Supplemental Figure 1. F2R S1PR1 GPR160 ELTD1 CD97 Supplemental Figure 2. A B brain heart lung liver kidney spleen testis skm Expression in mouse ssues glom rok Gprc5a Gapdh Human Protein Atlas RNAseq database Supplemental Figure 3. A Gprc5a Pdgfrb Merged CL CL Gprc5a CD31 Merged CL CL B C 1.4 * 1.2 mc 1 matrix 0.8 0.6 0.4 matrix 0.2 0 pod end 1 2 mes3 D Vector Gprc5a E 40 kD - - Gprc5a - acn Supplemental Figure 4. Control 12 month-old KO 12 month-old A B C D E F 1 2 0.8 * 1.5 score 0.6 m µ 1 0.4 0.2 Slits/ 0.5 Mesangial 0 0 Ctrl KO Ctrl KO Supplemental Figure 5. A B 1.2 Vector Gprc5a 1 * 0.8 0.6 * 0.4 * Normalized Density 0.2 0 pEGFR/tEGR pSmad/tSmad TGF-β1 C 1.8 siCON 1.6 siGprc5a * 1.4 * * 1.2 1 0.8 0.6 NormalizedDensity 0.4 0.2 0 pEGFR/tEGR pSmad/tSmad TGF-β1 Supplemental table 1. List of glomerulus-expressed GPCRs as detected by qPCR. Data shown as mean ± standard deviation (Glom=glomerulus, Rok=rest of kidney). GPCR Glom Rok Glom/Rok LPAR6 41892,11 ± 38478,89 1040,06 ± 1370,12 39,28 ELTD1 30275,64 ± 14085,26 33,23 ± 46,99 910,13 GPR116 24020,06 ± 7789,84 55,1 ± 47,75 434,96 PTH1R 15402,81 ± 17644,32 7521,01 ± 3264,57 1,05 CALCRL 14096,09 ± 3854,84 199,06 ± 222,52 69,81 HPRT1 13342,04 ± 10677,69 1824,77 ± 1767,23 6,31 S1PR5 9474,7 ± 10124,3 110,84 ± 29,54 84,48 LPHN2 8645,89 ± 914,74 256,04 ± 293,87 32,77 FZD1 8176,2 ± 4947,45 1321,97 ± 1311,91 5,18 CXCR4 7097,31 ± 4388,91 535,98 ± 640,28 12,24 GPR160 6446,59 ± 1550,07 4816,3 ± 5918,99 0,34 NPY1R 6177,74 ± 6282,76 209,64 ± 296,48 28,47 PTGER4 5323,66 ± 3789,51 179,13 ± 210 28,72 RXFP1 -

Detection of H3k4me3 Identifies Neurohiv Signatures, Genomic
viruses Article Detection of H3K4me3 Identifies NeuroHIV Signatures, Genomic Effects of Methamphetamine and Addiction Pathways in Postmortem HIV+ Brain Specimens that Are Not Amenable to Transcriptome Analysis Liana Basova 1, Alexander Lindsey 1, Anne Marie McGovern 1, Ronald J. Ellis 2 and Maria Cecilia Garibaldi Marcondes 1,* 1 San Diego Biomedical Research Institute, San Diego, CA 92121, USA; [email protected] (L.B.); [email protected] (A.L.); [email protected] (A.M.M.) 2 Departments of Neurosciences and Psychiatry, University of California San Diego, San Diego, CA 92103, USA; [email protected] * Correspondence: [email protected] Abstract: Human postmortem specimens are extremely valuable resources for investigating trans- lational hypotheses. Tissue repositories collect clinically assessed specimens from people with and without HIV, including age, viral load, treatments, substance use patterns and cognitive functions. One challenge is the limited number of specimens suitable for transcriptional studies, mainly due to poor RNA quality resulting from long postmortem intervals. We hypothesized that epigenomic Citation: Basova, L.; Lindsey, A.; signatures would be more stable than RNA for assessing global changes associated with outcomes McGovern, A.M.; Ellis, R.J.; of interest. We found that H3K27Ac or RNA Polymerase (Pol) were not consistently detected by Marcondes, M.C.G. Detection of H3K4me3 Identifies NeuroHIV Chromatin Immunoprecipitation (ChIP), while the enhancer H3K4me3 histone modification was Signatures, Genomic Effects of abundant and stable up to the 72 h postmortem. We tested our ability to use H3K4me3 in human Methamphetamine and Addiction prefrontal cortex from HIV+ individuals meeting criteria for methamphetamine use disorder or not Pathways in Postmortem HIV+ Brain (Meth +/−) which exhibited poor RNA quality and were not suitable for transcriptional profiling.